Day 1 of EWMA 2024



Day 1 of the European Wound Management Association (EWMA) exhibition. We have enjoyed catching up with friends and meeting new people interested in our Premium Cold Plasma medical device. Our Clinical Bibliography of 80+ clinical trials and studies puts us at the forefront of Cold Plasma Medicine for wounds, surgical site infections and medical dermatology. Visit us at booth G70 to learn more.
EWMA 2024

We look forward to exhibiting at the European Wound Management Association (EWMA) exhibition next week. Positioned at booth #G70, our Premium Cold Plasma medical device, the SteriPlas, will be featured to the public. Adtec Healthcare Cold Plasma company can boast of having very strong clinical trials data amongst a total of 80+ peer reviewed studies. Our medical device has proven anti-biofilm efficacy in chronic infections within wounds, surgical site infections and medical dermatology conditions.
Day 1 of the ISHLT


An exciting start to the ISHLT 2024 show. Our SteriPlas Premium Cold Plasma medical device is being widely spoken about throughout the sessions and we welcome you to visit our booth no.12 to learn more.
Adtec to exhibit at the ISHLT show

We are excited to be exhibiting at the ISHLT show next week. Our team will be onsite at booth no. 12 ready to welcome you and introduce you to our SteriPlas Premium Cold Plasma medical device used for the treatment of LVAD infections complicated with biofilm. Many of our customers will also be at the show who would be happy to give you a demonstration of our SteriPlas or to explain the strong clinical efficacy observed when using our medical device.
If you would like to secure a meeting in advance, please contact us at info@adtecplasma.com
Adtec joins the Innovation Platform at LSI 2024

We look forward to participating at the Innovation Platform at the @LSI USA 2024. Our CEO, Mary McGovern, will be in the Private Partnering Area between 18-22 March 2024 meeting with exclusive Medtech and Healthtech leaders to bring the rich content of the SteriPlas Premium Cold Plasma to them.
The SteriPlas is renowned for its proven clinical efficacy destroying chronic and complicated biofilm within infected wounds and surgical site infections. Our wide clinical bibliography of 80+ clinical trials and studies ranges from diabetic foot ulcers, LVAD infections, Sternal wound infections and so on.
Delivering a unique physical mode of action during treatment, the SteriPlas dense Cold Plasma ensures the total destruction of all bacterial species protected within biofilm which would otherwise have been resistant to antibiotics. Essentially, we are saving limbs !
We have documented the cost savings benefit due to be published this year across two separate papers focused on diabetic foot ulcers and sternal wound infections.
Our purpose of the LSI Innovation Platform attendance is to seek the potential of investment to help bring our medical device to new territories that have yet to benefit from what we can offer.
Cold Plasma is changing the norm of modern medicine.
Adtec visits Taiwan for SteriPlas expansion project




Who better to facilitate the expansion of the SteriPlas Premium Cold Plasma in the SE Asia market but the leading medical distributor, SG Biomedical. Adtec Healthcare Limited joins SG Biomedical in Taiwan to complete the introduction of three SteriPlas medical devices in the leading wound care hospitals.
Over 30 patients have so far been treated ranging from Diabetic Foot Ulcers, LVAD infections, Burn wounds, Vascular and Orthopaedic wounds and so on. All patients have had chronic infection with biofilm prior to being introduced to the SteriPlas. Some wounds have been non-healing for 4 years!
We are happy to report that over continued twice weekly use over the last two months, all patients are responding very well with most having been fully healed and no relapse of infection.
If you are within the Taiwan region and you are interested to learn more, please contact us so that we may introduce you to our partner.
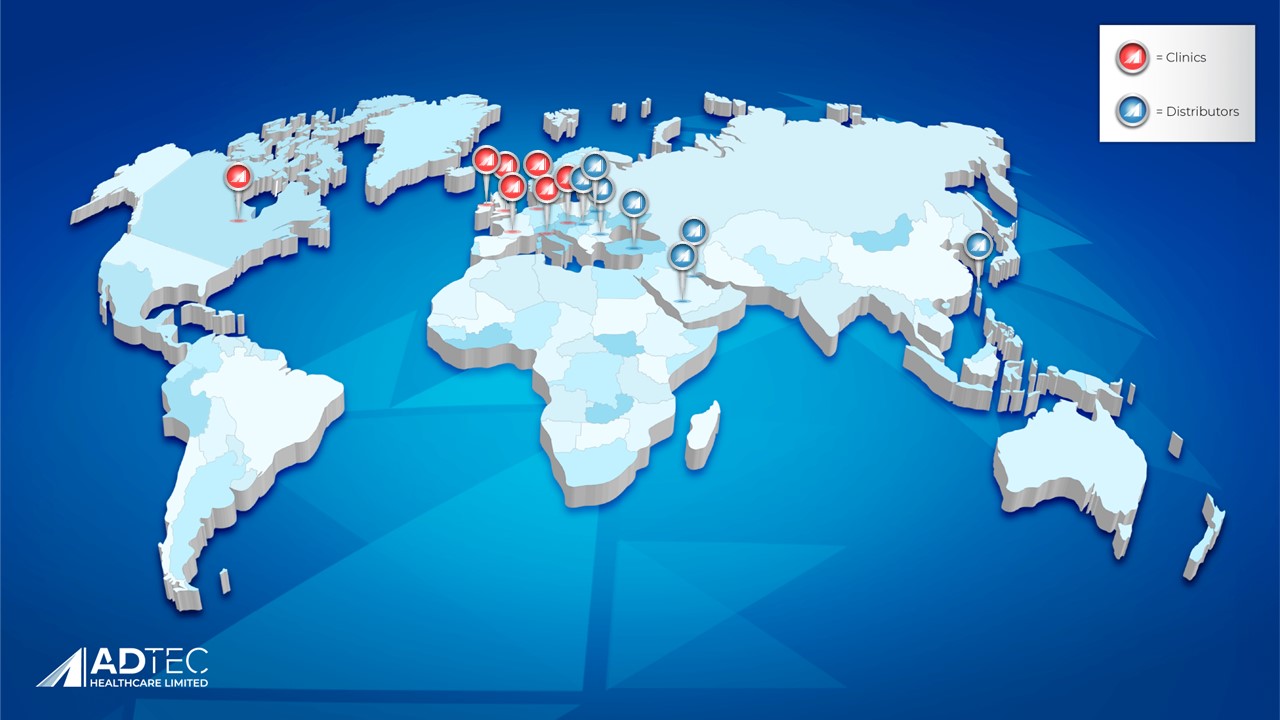
Adtec's World Map
Launching an innovative and unknown technology on the medical market has only been successful due to the many years of clinical evidence and trust that we have accrued. From being the first company worldwide to introduce Cold Plasma on wounds in clinical trials to now being the leading manufacturer behind the most effective Cold Plasma medical device, Adtec Healthcare Limited has the strongest experience on how to successfully combat infections stalled by biofilm.
Our global presence of the SteriPlas Premium Cold Plasma is increasing with multiple clinics and distributors endorsing our medical device for chronic infection management of wounds, surgical site infections and medical dermatology.
There are multiple clinics in each country that help to spread the word of the benefit that our medical device brings to non-healing infections. Unfortunately, there is not enough space on this small map to reflect this!
We have also significantly increased our Distributor portfolio into new countries who are rapidly bringing the SteriPlas to their clinics and customers, with plans to expand even further into new territories which we are currently working on.
To learn more about the successes of our dense and rich Cold Plasma, send us a message at info@adtecplasma.com. We are also keen to reach out to new distributors who also wish to bring our Cold Plasma to their territories.
Effective at deep and difficult to treat infection areas
The Premium Cold Plasma from our SteriPlas can kill all bacterial species, regardless of the resistance profile or if they are protected within biofilm. Due to the dense and rich Cold Plasma created, this includes treating difficult to reach areas such as LVAD drivelines, catheters and stoma infections.
Propelled by the gas flow, the Cold Plasma will travel down the gaps around the crevices of the infected driveline until it reaches the site of biofilm which is typically deep underneath the skin fold.
The enhanced physical mode of action of the SteriPlas is delivered quickly and effectively, eradicating bacteria which would otherwise have been resistant to conventional therapies such as antibiotics. These benefits are widely documented including the safety of having no side effects associated to our reliable and consistent Cold Plasma.
The adoption of the SteriPlas has been significantly increasing across the globe for LVAD infection treatments with promising results. To learn more about the SteriPlas, send us an email at info@adtecplasma.com.
Arab Health Exhibition 2024

We’re excited to be attending the Arab Health Exhibition next week to meet with our new partners across the Middle East. This year Adtec Healthcare Limited welcomes the expansion of our Cold Plasma to the MENA region, facilitated by our Distributors in their leading fields of wound care and surgical site infections.
If you would like to secure your meeting with us during the Arab Health show, please send us an email at info@adtecplasma.com
The Power of Going Large

The SteriPlas boasts a wide clinical bibliography of clinical trials and publications, documenting the strong clinical efficacy coupled with no side effects reported. This benefits to patients suffering from non-healing wounds infected with biofilm whereby quick 2 minute treatment sessions are able to destroy all bacteria present and heal patients in as quick as 1 week.
To make such a statement as the above requires strong, reputable, and quality-driven results which we are thankful to have. But to collect these results and optimise the technical build of the SteriPlas has taken many years. From our first ever clinical trial on wounds approximately two decades ago to today, the SteriPlas has had its fair share of changes.
Whilst there have been multiple beneficial changes, the overall size of the SteriPlas has remained the same for a crucial reason. The Steriplas is the size it is to produce the optimum plasma for clinical efficacy using microwave argon plasma. Whilst Cold Plasma can still be generated with various technologies, it cannot be done in the same dense and rich manner delivering antibiofilm results. Crucial components to ensure the consistency and reliability may be lost. Most cold plasmas will have antibacterial efficacy against planktonic strains in in-vitro non-competing environments, but chronic wounds are a complex topography of biofilm, exudate, slough, living and necrotic tissue.
Adtec Plasma Technology has developed many Cold Plasma devices since its existence from small pens, portable shoulder strap machines to large medical devices. Our legacy device, the SteriPlas, remains the clinical leader in Cold Plasma medicine for wounds, surgical site infections and medical dermatology conditions.