SteriPlas to be presented at the DFSG conference
Adtec looks forward to supporting Ms Jemma Cruickshank, Antimicrobial Pharmacy Technician from Kettering General Hospital NHS Foundation Trust, at the Diabetic Foot Study Group (DFSG) conference in September.
Jemma has been invited to present the “Retrospective review of the use of argon cold plasma therapy in non-healing diabetic foot ulcers over a 3-year period within a DGH Diabetic Foot MDT Service” at the conference.
Her presentation will include the strong benefits of the SteriPlas on chronic and large diabetic foot ulcers prone to biofilm infections.
The abstract for this presentation can be found here: https://distribute.m-anage.com/from.storage?image=erIsXlaDuNOv64Tv4JyKY6XW3cI1HigkZNgT5cOgYv7Vzt_UHvGbDPesijzVroVO0
For more information about our SteriPlas Premium Cold Plasma medical device, send us an email info@adtecplasma.com
Is all Cold Plasma created equal?

There is no doubt a wide variety of Cold Plasma medical devices currently on the market and new Cold Plasma designs often being released by startup companies. But how do these devices compare in efficacy, strength, reliability, and performance?
To say all Cold Plasmas are the same, which some companies (shockingly) do, would be farfetched and misleading. This is like comparing a race between a sports car and a bicycle or saying all liquids or gases are the same – the product and plasma components are technically very different.
When Adtec first developed Cold Plasma and carried out the first ever clinical trials on wounds many years ago, our data showed the remarkably strong and reputable results that we still deliver today. This is exclusively for our range of Adtec Cold Plasma medical devices. Our data is often cited by other Cold Plasma companies as their own medical devices lack strength in efficacy and performance in clinical data.
The important questions to raise are:
- What is the frequency of Cold Plasma applications per week to achieve full healing?
- How deep can the Cold Plasma penetrate?
- How quick can the Cold Plasma be applied?
- What is the delivery method of Cold Plasma?
- Is Cold Plasma limited to acute and superficial wounds or can it treat deep, chronic wounds with biofilm?
- What is the clinical evidence for the product itself and not cold plasma in general?
Whilst there are some Cold Plasma medical devices that do not satisfy the requirements met by a wound clinician, the SteriPlas does remain the leader and favorable choice in the Cold Plasma medical market. A once per week treatment time of 2 minutes per 12cm2 has been shown to accelerate healing in chronic, problematic, and non-healing wounds with biofilm presence. This cannot be said for all other Cold Plasma devices which recommend treatment once per day and are only indicated for acute, small, and superficial wounds certainly with no biofilm presence.
Due to its overall larger size which houses crucial components ensuring a consistent, reliable and therapeutic Cold Plasma, the SteriPlas is the only microwave-energy, Cold Argon Plasma medical device which delivers deep, dense and rich Cold Plasma able to kill all bacterial species embedded within biofilm. We can boast that no side effects have been reported with our Cold Plasma medical device since the history of its making.
For more information about the SteriPlas and how we have changed the way that wounds are healed, please visit our page: https://adtechealthcare.com/cold-plasma-wound-treatment/
80 Peer-reviewed Clinical Studies

Can the SteriPlas:
Kill all forms of bacteria? YES ✔
Kill bacteria protected within biofilm? YES ✔
Operate safely with no side effects? YES ✔
Treat infected wounds in as quick as 2 minutes? YES ✔
Ready to learn more? Visit our website: https://adtechealthcare.com/cold-plasma-wound-treatment/
Our 80 peer-reviewed clinical trials and publications illustrate why our SteriPlas remains the leader for Cold Plasma with anti-biofilm efficacy
#medicaldistributor #MEDICA2023 #ABHI #Plasma #investorswanted #investor
Day 1 of Medica


Day 1 of the MEDICA Trade Fair was a success. Interacting with many distributors and clinicians with positive interest in our promising Premium Cold Plasma. We encourage you to visit our booth again today to see why the SteriPlas is revolutionising the global wound care market.
Don't forget to attend our presentation today from 11:55 - 12:15 at the ABHI stand. We will be presenting the strong clinical evidence of the SteriPlas Premium Cold Plasma used for wounds, Surgical Site Infections and medical dermatology in the UK Theatre in Hall 16, J48.
Medica Trade Fair 2023

We are delighted to be joining The Association of British HealthTech Industries (ABHI) and UK Healthcare Pavilion at the Medica Trade Fair next week, showcasing the strengths of UK HealthTech alongside 40 of the UK’s leading industry players. Come visit us at booth J60.3, Hall 16 and discover the best of the UK.
Adtec Microwave Argon Plasma has proven clinical efficacy in chronic infection management compared to standard care.
Adtec’s patented microwave argon plasma was designed specifically to create a denser safe plasma to manage infection with proven clinical efficacy over standard treatments. Our Plasma blend is effective at treating bacteria enclosed within biofilm and reaching hard to treat areas e.g. along drivelines. This clinical evidence has been presented at conferences and published in over 80 peer-reviewed publications.
The recent clinical evidence featured in Mr Thomas Schlöglhofer’s data illustrates the advantages of our SteriPlas medical device for deep LVAD infections. This showed Adtec Cold Plasma had 81% efficacy at wound management vs 47.1% efficacy with standard of care.
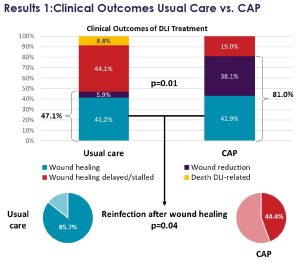
“Cold Atmospheric Plasma Therapy as an Effective Treatment of Left Ventricular Assist Device Driveline Infections” at the EACTS European Association for Cardio-Thoracic Surgery conference in Vienna (October 2023) presented by Mr Thomas Schlöglhofer, the Department of Cardiac Surgery, Medical University of Vienna.
Clinical Trials conducted using Adtec microwave argon plasma results include a 73.5% reduction in bacterial load with standard of care plus plasma treatment compared to a 50% reduction with standard of care alone. This represents a 47% increase in bacterial load reduction with SOC and plasma compared to SOC. All 24 patients received standard wound care to all wounds; 22 patients also received systemic antibiotics (92%).
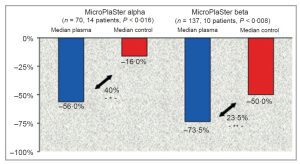
Successful and Safe Use of 2 Min Cold Atmospheric Argon Plasma in Chronic Wounds: Results of A Randomized Controlled Trial. Isbary, G., et al British Journal of Dermatology, 2012. 167(2)
TFDA Approves SteriPlas in Taiwan
It is with great pleasure that we announce the SteriPlas Cold Plasma medical device is now an approved product in Taiwan, making it the first Cold Plasma medical device to be issued in the country. Adtec Healthcare Limited owes its thanks and praises to our exclusive distributor in Taiwan, SG Biomedical, whom without their skills in Regulatory and with the TFDA, none of this would have been possible.
The SteriPlas is already renowned in Taiwan for its strong clinical efficacy using premium and dense Cold Plasma and is set for distribution across the leading hospitals dealing with wound care. This is set to start within the coming months. If you are a health care professional in Taiwan that manages chronic wounds, we humbly request that you contact SG Biomedical who can guide you on the strong antibacterial benefits of the SteriPlas for diabetic foot ulcers, LVAD infections, sternal wounds and many more.
http://www.sghlds.com/index.php/tw/

SteriPlas Cold Plasma to be presented at EACTS 2023

We look forward to Mr Thomas Schlöglhofer’s presentation at the upcoming European Association for Cardio-Thoracic Surgery (EACTS) conference in Vienna on Fri 6th Oct. Thomas will present, “Cold Atmospheric Plasma Therapy as an Effective Treatment of Left Ventricular Assist Device Driveline Infections” which will feature the advantages of our SteriPlas Cold Plasma medical device for deep LVAD infections treated at the Department of Cardiac Surgery, Medical University of Vienna.
Adtec presents at the LSI today

It was a pleasure to present at the LSI - Life Science Intelligence in Barcelona today. The successes of Adtec Healthcare Ltd and our Premium Cold Plasma medical device, the SteriPlas, were presented to the audience with much interest.
If you would like to know more about our strong clinical efficacy and antibiofilm properties for chronic wounds and infections, send us an email at info@adtecplasma.com
First day at the LSI


An epic start to the first day at LSI - Life Science Intelligence in Barcelona.
It has been a pleasure to interact with professionals sharing the same interest in tackling strains on the global wound care market. The benefits that the clinically proven SteriPlas can bring to significantly reduce bed occupancy, chronic infections and the financial burden rates have been demonstrated.
We look forward to presenting more about this on the 21st Sept and encourage you to meet with us whilst at LSI to learn more about our Premium Cold Plasma medical device for chronic wounds, surgical site infections and medical dermatology conditions.








