What makes our SteriPlas safe and effective?

What makes our SteriPlas Premium Cold Plasma safe and effective?
Adtec has been the global leader of Cold Plasma medicine, having developed Cold Plasma products for over 20 years and being the first company worldwide to conduct clinical trials on wounds. In total, Adtec boasts over 80+ clinical trials and studies with no side effects reported.
Whilst there are many forms of Cold Plasmas on the market, our SteriPlas Cold Plasma is dense and slowly propelled towards the treatment site enabling bacterial destruction in deep and hard to reach infection areas. The argon Cold Plasma is microwave, 2.45GHz generated using crucial components within the body of the medical device. This is why it is larger in size but at the advantage of delivering reliable and consistent results and easily tackles complicated biofilm issues within chronic infections unlike the other variations of Cold Plasma on the market. This is also why our clinical evidence is often cited by other Cold Plasma manufacturers as our promising results far outweigh the results achieved by Cold Plasma devices with different plasma designs.
To learn more about our strengths and benefits, contact us at info@adtecplasma.com
Leading the way in Cold Plasma Medicine

The antibiofilm properties of the SteriPlas has been widely documented across our 80+ clinical trials and studies. It is being widely used to treat a multitude of infection conditions such as Diabetic Foot Ulcers, Sternal Wounds and LVAD infections in the UK, EU and SE Asia. Helping to significantly reduce morbidity, mortality, amputation and bed occupancy rates, the SteriPlas has been transforming modern medicine by simplifying and accelerating the way these chronic and non-healing wounds are treated. We have also documented the significant cost saving benefits of using the SteriPlas Premium Cold Plasma all with the added benefit of no side effects due to its safe, microwave 2.45GHz argon plasma generation.
To learn more about the benefits we can bring you, contact us at info@adtecplasma.com
Adtec visits Taiwan for SteriPlas expansion project




Who better to facilitate the expansion of the SteriPlas Premium Cold Plasma in the SE Asia market but the leading medical distributor, SG Biomedical. Adtec Healthcare Limited joins SG Biomedical in Taiwan to complete the introduction of three SteriPlas medical devices in the leading wound care hospitals.
Over 30 patients have so far been treated ranging from Diabetic Foot Ulcers, LVAD infections, Burn wounds, Vascular and Orthopaedic wounds and so on. All patients have had chronic infection with biofilm prior to being introduced to the SteriPlas. Some wounds have been non-healing for 4 years!
We are happy to report that over continued twice weekly use over the last two months, all patients are responding very well with most having been fully healed and no relapse of infection.
If you are within the Taiwan region and you are interested to learn more, please contact us so that we may introduce you to our partner.
Does more power lead to enhanced wound care treatment?

In simple terms, the answer is yes. To look more in depth at the question we need to understand how the Cold Plasma is generated and the mechanics behind it. The SteriPlas boasts a large 12cm2 treatment area which fully covers typical chronic wounds expected in wound clinics. There are of course much larger wounds than 12cm2 but thanks to the mechanics behind the SteriPlas it only requires a 2 minute treatment time for each 12cm2 area it covers.
There are some Cold Plasma devices that claim a 100cm2 treatment area but how does the power behind it compare to the SteriPlas? It is without question, the SteriPlas is one of the largest Cold Plasma medical devices on the medical market and it is strategically large for a reason. It is the only Cold Plasma medical device able to produce a deep, dense, and rich Cold Plasma. To produce repeatable and consistent results with antibiofilm properties, there are a series of crucial internal components in the SteriPlas that collectively work to perform this including the 200W 2.45GHz microwave generator, plasma ignition control, mass flow controller, arm to hold plasma treatment head in place during use, control system linked to interlock and alarms, cooling system and safety circuits.
Smaller Cold Plasma devices with larger treatment areas generate different plasmas. Whilst some reduction of bacterial load may be observed with their use, it is strictly limited to superficial and small wounds certainly with no biofilm presence. To combat biofilm within wounds, the Cold Plasma does need to be deeply penetrating and to do this more power and plasma density is certainly required.
The SteriPlas, by default, is designed to target deep, chronic and severe infections with biofilm complications within the wound. Peer reviewed clinical evidence for smaller devices with larger treatment areas are still limited.
The SteriPlas boasts a wide clinical bibliography of 80+ peer reviewed clinical trials and studies. It is the leader in the Cold Plasma medical market for a reason.
To learn more about our medical device, send us an email info@adtecplasma.com
Is all Cold Plasma created equal?

There is no doubt a wide variety of Cold Plasma medical devices currently on the market and new Cold Plasma designs often being released by startup companies. But how do these devices compare in efficacy, strength, reliability, and performance?
To say all Cold Plasmas are the same, which some companies (shockingly) do, would be farfetched and misleading. This is like comparing a race between a sports car and a bicycle or saying all liquids or gases are the same – the product and plasma components are technically very different.
When Adtec first developed Cold Plasma and carried out the first ever clinical trials on wounds many years ago, our data showed the remarkably strong and reputable results that we still deliver today. This is exclusively for our range of Adtec Cold Plasma medical devices. Our data is often cited by other Cold Plasma companies as their own medical devices lack strength in efficacy and performance in clinical data.
The important questions to raise are:
- What is the frequency of Cold Plasma applications per week to achieve full healing?
- How deep can the Cold Plasma penetrate?
- How quick can the Cold Plasma be applied?
- What is the delivery method of Cold Plasma?
- Is Cold Plasma limited to acute and superficial wounds or can it treat deep, chronic wounds with biofilm?
- What is the clinical evidence for the product itself and not cold plasma in general?
Whilst there are some Cold Plasma medical devices that do not satisfy the requirements met by a wound clinician, the SteriPlas does remain the leader and favorable choice in the Cold Plasma medical market. A once per week treatment time of 2 minutes per 12cm2 has been shown to accelerate healing in chronic, problematic, and non-healing wounds with biofilm presence. This cannot be said for all other Cold Plasma devices which recommend treatment once per day and are only indicated for acute, small, and superficial wounds certainly with no biofilm presence.
Due to its overall larger size which houses crucial components ensuring a consistent, reliable and therapeutic Cold Plasma, the SteriPlas is the only microwave-energy, Cold Argon Plasma medical device which delivers deep, dense and rich Cold Plasma able to kill all bacterial species embedded within biofilm. We can boast that no side effects have been reported with our Cold Plasma medical device since the history of its making.
For more information about the SteriPlas and how we have changed the way that wounds are healed, please visit our page: https://adtechealthcare.com/cold-plasma-wound-treatment/
Why Bigger is Better?

The SteriPlas has been widely documented in clinical trials and publications for its strong antibacterial efficacy and accelerated healing properties. Being the first medical device worldwide to show just how effective Cold Plasma can be from clinical trials on wounds, it is still the only medical device covering a larger 12cm2 treatment area using microwave-energy, safe, reputable, and consistent Cold Plasma. It is also the only medical device that can treat wounds within this size for only 2 minutes to achieve antibiofilm efficacy with no reported side effects.
So how does it compare to a Cold Plasma Pen?
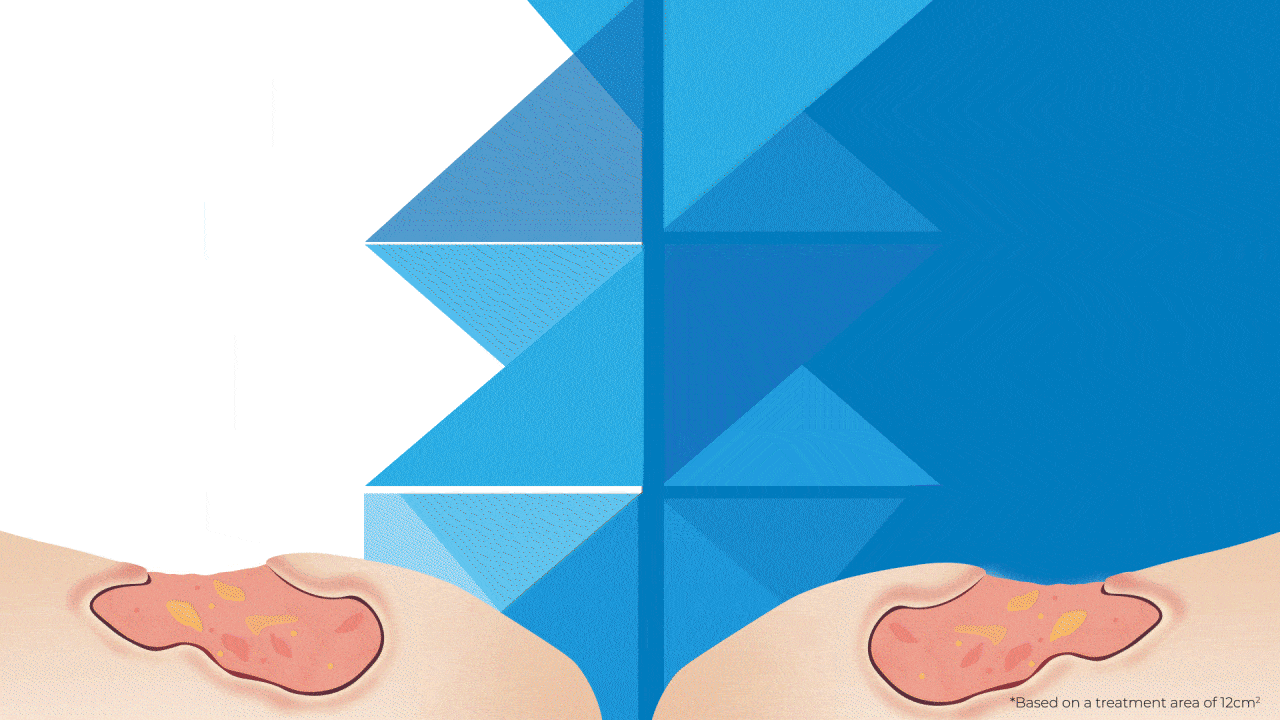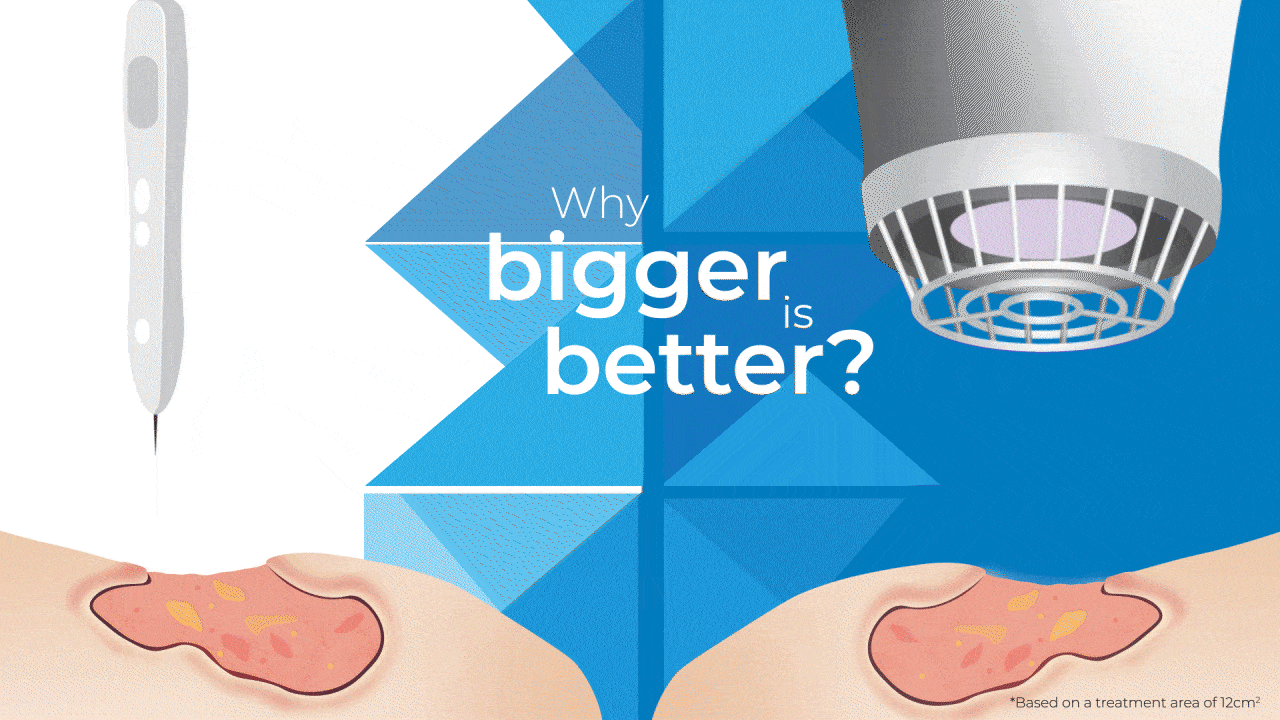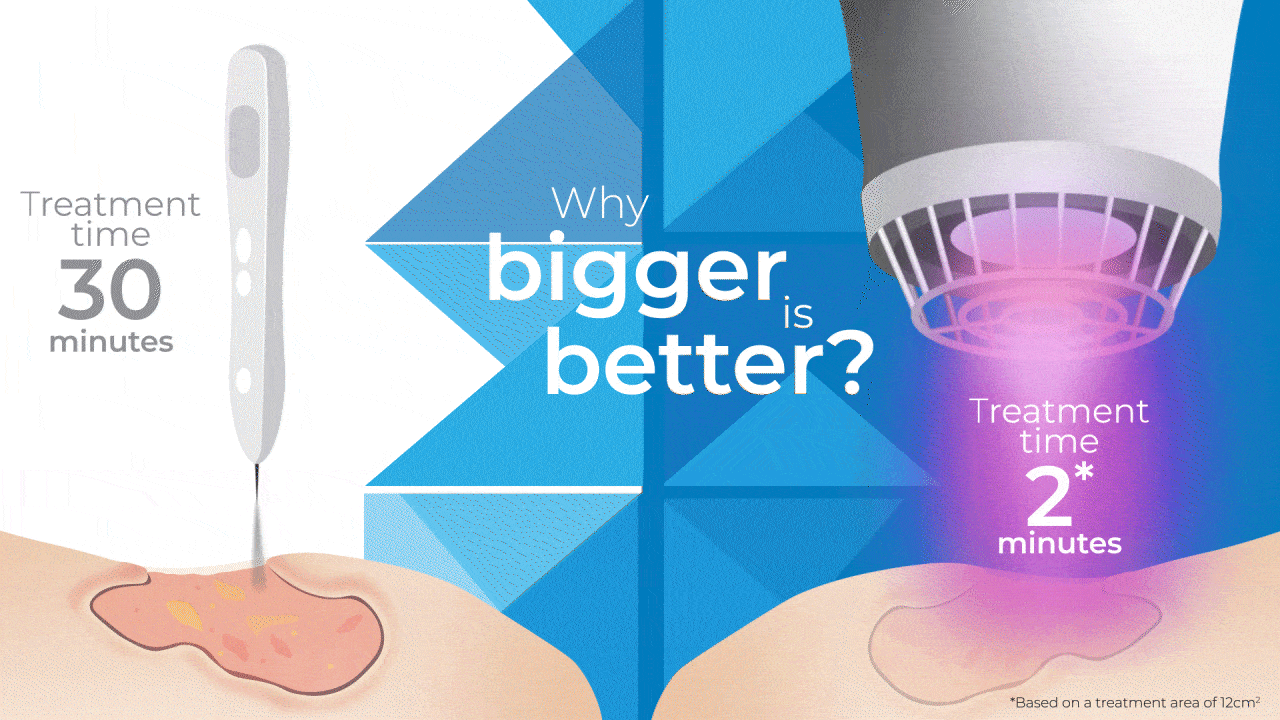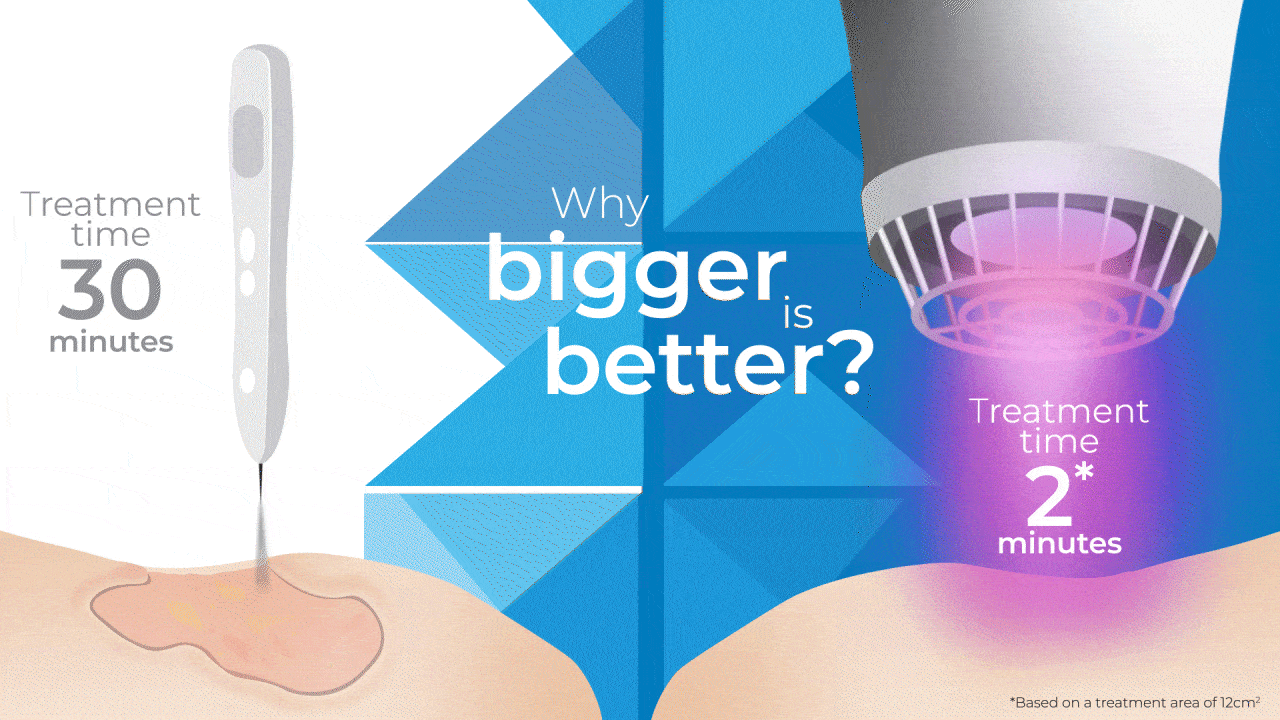
As useful as Plasma pens can be for very small and superficial wounds without biofilm, 2 minutes is required for the treatment area equivalent to a few mms.
This means to treat a large and deep wound that the SteriPlas can do in 2 minutes (hands-free), then Plasma Pens would require in excess of 30 minutes with handheld use to reduce the bacterial load present in the same wound area . The SteriPlas is the only medical device with antibiofilm efficacy due to its dense and deep penetration capabilities making it the leader in Cold Plasma medicine.
80 Peer-reviewed Clinical Studies

Can the SteriPlas:
Kill all forms of bacteria? YES ✔
Kill bacteria protected within biofilm? YES ✔
Operate safely with no side effects? YES ✔
Treat infected wounds in as quick as 2 minutes? YES ✔
Ready to learn more? Visit our website: https://adtechealthcare.com/cold-plasma-wound-treatment/
Our 80 peer-reviewed clinical trials and publications illustrate why our SteriPlas remains the leader for Cold Plasma with anti-biofilm efficacy
#medicaldistributor #MEDICA2023 #ABHI #Plasma #investorswanted #investor
Visit us at booth J60.3, Medica 2023

Next week we’ll be exhibiting at the Medica Trade Fair 2023 exhibition with the ABHI and UK Healthcare Pavilion. You can find us at booth J60.3 in Hall 16. Be sure to stop by to see our SteriPlas Premium Cold Plasma medical device live in action and ready to demonstrate its strong clinical efficacy for wounds, surgical site infections and medical dermatology conditions.
Adtec Healthcare is also eager to meet with new medical distributors to continue to expand the reach of our Cold Plasma globally. If you are a distributor and willing to learn about the strong cost saving benefits and accelerated healing of chronic wounds with biofilm please be sure to pay us a visit.
Medica Trade Fair 2023

We are delighted to be joining The Association of British HealthTech Industries (ABHI) and UK Healthcare Pavilion at the Medica Trade Fair next week, showcasing the strengths of UK HealthTech alongside 40 of the UK’s leading industry players. Come visit us at booth J60.3, Hall 16 and discover the best of the UK.
TFDA Approves SteriPlas in Taiwan
It is with great pleasure that we announce the SteriPlas Cold Plasma medical device is now an approved product in Taiwan, making it the first Cold Plasma medical device to be issued in the country. Adtec Healthcare Limited owes its thanks and praises to our exclusive distributor in Taiwan, SG Biomedical, whom without their skills in Regulatory and with the TFDA, none of this would have been possible.
The SteriPlas is already renowned in Taiwan for its strong clinical efficacy using premium and dense Cold Plasma and is set for distribution across the leading hospitals dealing with wound care. This is set to start within the coming months. If you are a health care professional in Taiwan that manages chronic wounds, we humbly request that you contact SG Biomedical who can guide you on the strong antibacterial benefits of the SteriPlas for diabetic foot ulcers, LVAD infections, sternal wounds and many more.
http://www.sghlds.com/index.php/tw/