First ever recorded study for CAP on Pediatric Cannula infections

🚨 Exciting News! 🚨
We are thrilled to share a groundbreaking study by Dr. Schlöglhofer, recently published in the Artificial Organs journal from Wiley. This study highlights the remarkable safety and efficacy of using SteriPlas Premium Cold Plasma for managing infections on Berlin Heart EXCOR cannulas.
🌟 This is the first publication to demonstrate the potential of CAP therapy for treating infections and inflammation in pediatric patients with paracorporeal pulsatile VADs.
Key findings show that SteriPlas treatments:
- Do not alter the surface structure of cannulas, as confirmed by SEM micrographs.
- Lead to significant improvements in DESTINE wound staging.
- Drastically reduce bacterial load and inflammatory markers.
- Achieve these results with no observed side effects.
This study marks an exciting step forward in infection management for pediatric VAD patients. To read the full publication, visit: Wiley Library.
Exhibiting at the EUMS 2024 show


Dr Schlöglhofer presents the SteriPlas at ESAO 2024




A huge congratulations to Dr Thomas Schlöglhofer for his presentation at the European Society for Artificial Organs 2024 conference.
His presentation, “Cold Atmospheric Plasma Therapy as a Novel Treatment for Berlin Heart EXCOR Pediatric Cannula Infections” features the use of our SteriPlas Premium Cold Plasma for infection management on Berlin Heart EXCOR cannulas in Pediatric patients.
We thank Dr Schlöglhofer for his work towards this study and for recommending the SteriPlas as the only Cold Plasma device for the infection management of Berlin Heart EXCOR products.
Presentation at the DFSG 2024



We enjoyed watching Jemma present on behalf of Kettering General Hospital NHS for the SteriPlas treatment of chronic diabetic foot ulcers at the Diabetic Foot Study Group (DFSG). Our Premium Cold Plasma has proven efficacy and anti-biofilm properties essential for the healing of these complex wounds.
Day 2 of the DFSG 2024


Glad to be at the Diabetic Foot Study Group (DFSG) conference catching up with existing customers and to those new who have learnt the benefits of our SteriPlas Premium Cold Plasma. We look forward to the Kettering NHS presentation later today featuring the SteriPlas efficacy for chronic Diabetic Foot Ulcers.
Exhibiting at the DFSG 2024

SteriPlas combats Antimicrobial Resistance (AMR)

In 2016, the NHS identified the need to find clinically and cost-effective interventions for patients with diabetic foot disease as a high priority (College of Podiatry, 2019). This urgency is compounded by the rapidly rising incidence of antimicrobial resistance (AMR), a particular concern in people with DFU as infections are the leading cause of amputation (Hurlow et al 2018).
The SteriPlas has the ability to kill all forms of bacteria regardless if they are Gram-positive or Gram-negative or if they are protected within biofilm. It is a broad spectrum, topical antibacterial medical device that delivers consistent Premium Cold Plasma therapy directly at the site of infection. By eliminating bacteria that prevents the body from healing, it significantly reduces the antimicrobial resistance rates and prevents amputations from occurring.
Our proven clinical trials and studies have placed the SteriPlas as the leading Cold Plasma medical device in the market.
For more information about our strong clinical efficacy and #HealthEconomics, contact us at info@adtecplasma.com
SteriPlas proven to heal patients faster
“In cases with biofilm complications, CAP patients had an average wound area reduction of 44% whereas patients from the Control group had an average increase of 167%.”
Diabetic foot ulcers complicated with biofilm are already prone to poor arterial flow, therefore, the delivery of antibiotics to a complicated site already infected with multi-resistant bacteria may pose little to no help in healing the wound. This unfortunately can lead to amputations and further complications.
The SteriPlas benefits significantly to antibiotics due to its unfailing physical mode of action delivery during treatment. Multi-resistant bacteria, whether embedded and protected in biofilm, is quickly destroyed in as quick as 2 minutes. The treatment is delivered directly at the site of infection, is painless, contact-free, and most importantly has no side effects. As bacteria is destroyed so easily with our Premium Cold Plasma therapy, patients are healed and relieve the costs burden associated to hospitals.
“The SteriPlas is a cost and clinically effective treatment option for diabetic patients with infected chronic lower leg wounds in the absence of significant peripheral arterial disease.”
For more information about our strong clinical efficacy and #HealthEconomics, contact us at info@adtecplasma.com
SteriPlas proven to lower antibiotic usage
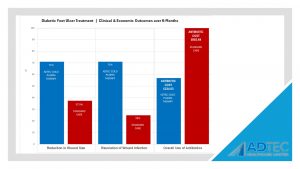
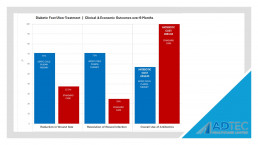
“Patients in the CAP treatment group were more likely to achieve reduction in wound size (71% vs 37.5%), resolution of wounds infection (71% vs 25%) and lower overall use of antibiotics (57% vs 100%).”
Our Health Economics data further demonstrates why the SteriPlas Premium Cold Plasma is the leading medical device for chronic diabetic foot ulcers. Continuing to relieve the cost burden associated to hospitals dealing with infection, the SteriPlas has been transforming how modern medicine works. Many users have changed their treatment guidelines to incorporate the SteriPlas and replace existing therapies that failed to work as well as the SteriPlas does.
For more information about our strong clinical efficacy and Health Economics, contact us at info@adtecplasma.com
Cheaper and Faster than Conventional Therapies
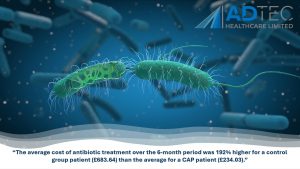
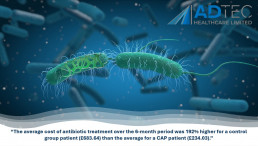
“The average cost of antibiotic treatment over the 6-month period was 192% higher for a control group patient (£683.64) than the average for a CAP patient (£234.03).”
The annual National Health Service (NHS) cost of wound management in the UK for 2017/18 was GB£8.3 billion. Of this, £5.6 billion was spent on managing unhealed wounds (Guest et al., 2020), including diabetic foot ulcers (DFUs) (Everett and Mathioudakis,2018). In 2014-2015 costs for ulceration and amputation in people with diabetes (PWD) was estimated at between £837 million and £962 million with more than 90% of expenditures related to ulceration (Kerr et al 2019).
In the USA, about 38 million Americans have diabetes, and each year a staggering 154,000 Americans will suffer amputations, roughly 80% of which will be the result of complications from diabetes. Their life expectancy following this procedure is five years.
Our mission has always been to revolutionise the global infection market. Bringing an innovative technology which aims to disrupt the conventional infection management market by delivering a more reliable, faster, and effective means of treatment. The SteriPlas has been doing exactly that since its launch many years ago and has expanded to cover the UK, EU and SE Asia with more plans to expand into new territories bringing the benefits to others who have yet to use our medical device.
For more information about our strong clinical efficacy and Health Economics, contact us at info@adtecplasma.com