ToGCPiCPT

Congratulations to our Business Development Manager, Jeiram, for his published chapter in the “Textbook of Good Clinical Practice in Cold Plasma Therapy”. This prestigious book houses powerful material from esteemed authors in the cold plasma medicine field.
Jeiram’s chapter, “SteriPlas® and PlasmaTact®”, discusses the importance of Adtec Healthcare’s influence in the cold plasma medicine field from being the first company ever to conduct cold plasma clinical trials on wounds and to our modern day clinical efficacy in wounds, surgical site infections and medical dermatology skin conditions.
You can view this chapter here: https://doi.org/10.1007/978-3-030-87857-3_18
For more information about our cold plasma medical and non-medical devices, please contact us at info@adtecplasma.com
The Safety and Reliability of our Cold Plasma

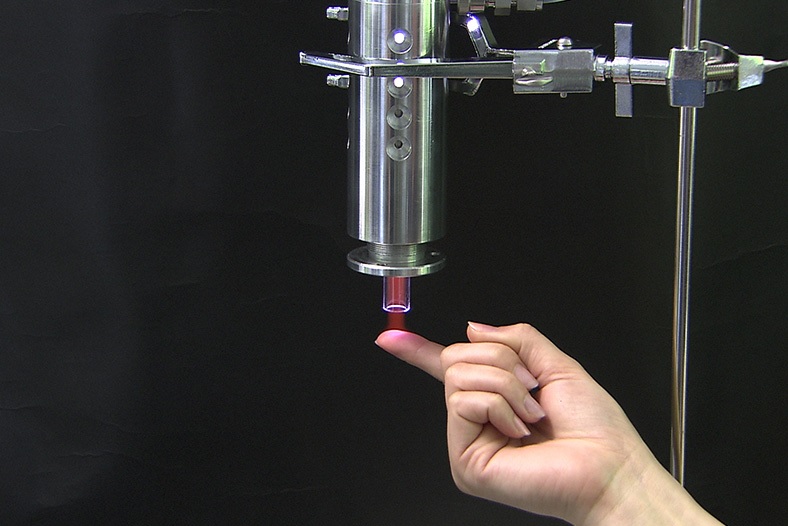
Is our cold plasma safe?
Yes!
As you can see from the photo, our Adtec technician demonstrates touching the cold plasma jet.
An extensive list of practical tests and experiments were conducted during our first stage, pre clinical studies and ongoing post-market studies. This allowed us to carefully develop a cold plasma medical device that could bring all the antibacterial benefits but at the same time remain painless and contact-free so that it could be well tolerated in patient treatments. We were after all the first company worldwide to introduce cold plasma treatment on wounds demonstrated in our clinical trials.
As cold plasma can be generated through a variety of ways, they are also dependent on the plasma source design and surrounding environment. The components of plasma include reactive oxygen and nitrogen species and UV that need to be controlled to ensure they are within safe levels for medical use. Some plasma devices may also deliver fluctuating inconsistent cold plasma output as they are sensitive to changes in humidity and temperature in the treatment room. This may often result in undesirable side effects.
Because the Adtec SteriPlas is a low energy, microwave-powered, cold atmospheric argon plasma medical device this means its safety remains the main priority in line with delivering repeatable and successful results. It is also the reason why we are proud to state our medical device has NO side effects associated to it.
Our patented 6 electrode Cold Plasma Torch

The Adtec SteriPlas features a patented 6 electrode cold plasma torch. It’s proven efficacy for the destruction of all forms of bacteria including multi-resistant bugs makes it an alternative to antibiotic therapy for the treatment of wounds, surgical site infections and medical dermatology skin conditions. This is due to the unique physical mode of action delivered with our cold plasma as opposed to the chemical mode of action demonstrated with antibiotics. Broader studies have shown that antimicrobial resistance is unlikely to be generated with cold plasma treatment because of this.
A huge collection of our peer reviewed publications documents the clinical efficacy and safety of our medical device across a broad range of conditions including diabetic foot ulcers, left ventricular assist device (LVAD) infections, deep sternal wound infections, actinic keratoses and acne.
To learn more about our medical device or to obtain one in your clinic practice, please contact us at info@adtecplasma.com
Adtec Healthcare's Conference and Exhibition attendance dates for 2022
As we look forward to a promising year ahead, please find the dates of our conference and exhibition participation for 2022:
Society for Cardiothoracic Surgery (SCTS) Annual Meeting – 8-10 May 2022
Malvern Diabetic Foot Conference – 11-13 May 2022
European Wound Management Association (EWMA) – 23-25 May 2022
Diabetic Foot Study Group (DFSG) – 16-18 September 2022
We hope to see you at either of these conferences and as always, we will have our flagship medical device, the Adtec SteriPlas, on demonstration ready for you to test and to see all the benefits linked to our cold plasma strengths for wounds, surgical site infections and medical dermatology.

![]()


The first cold plasma medical device to treat Scleroderma

We congratulate Dr Stephanie Ardnt and Prof. Sigrid Karrer from the University Hospital Regensburg for their recent publication, “The Anti-Fibrotic Effect of Cold Atmospheric Plasma on Localized Scleroderma In Vitro and In Vivo”.
This publication features the use of our Adtec SteriPlas and shows strong efficacy for the treatment of Scleroderma, which up to now has not yet been evaluated by CAP. The extensive study documents significantly reduced dermal thickness and collagen deposition as well as a decrease in both alpha smooth muscle actin-positive myofibroblasts and CD68-positive macrophages in the affected skin in comparison to untreated fibrotic tissue. This study provides the first evidence for the successful use of CAP for treating LS and may be the basis for clinical trials including patients with LS.
https://doi.org/10.3390/biomedicines9111545
Quick results achieved with the Adtec SteriPlas
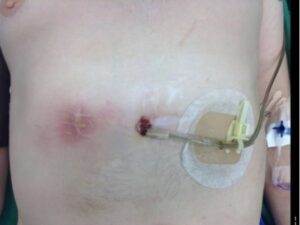
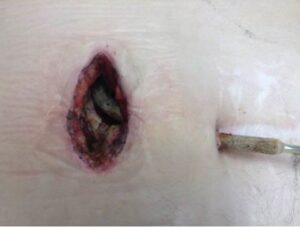
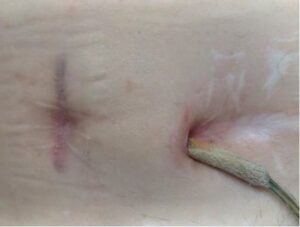
How often do LVAD patients with infection need treatment with the Adtec SteriPlas?
We recommend a minimum treatment of twice per week. This applies to both outpatients in day clinics and inpatients who have been admitted into the wards.
The treatment time itself is as quick as 3 minutes. During these short minutes, the components of cold plasma quickly bombard the cellular structure of bacteria to create micropores which allows access to the microbial DNA for total destruction. This applies to all forms of bacteria regardless of their resistance profile or if they are protected within biofilm.
How quick have patients with LVAD infections healed?
Our clinical evidence has shown patients have healed in as quick of two weeks of treatment. These are patients who have failed previous therapeutic attempts with conventional treatment methods.
Promising, consistent and reliable results achieved with the Adtec SteriPlas
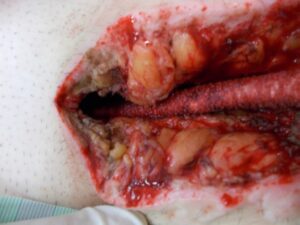
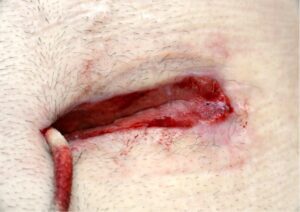
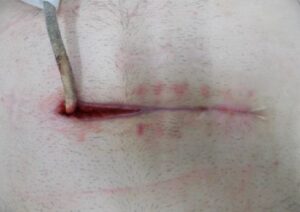
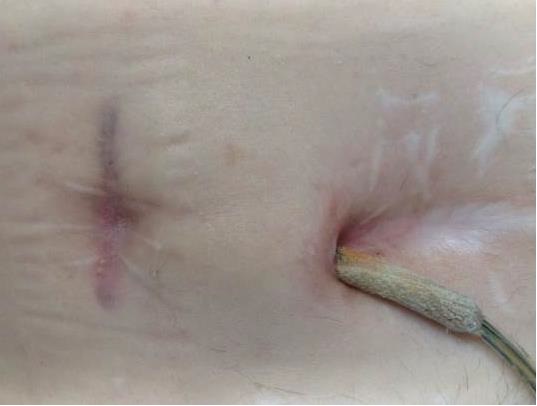
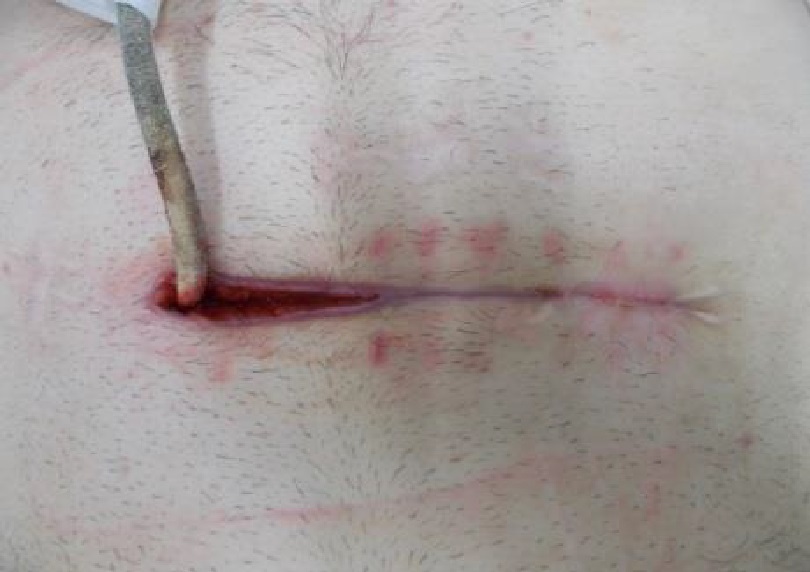
Whilst Left ventricular assist devices (LVADs) have become essential mechanical pumps for patients who have had end stage heart failures, they are also prone to infections especially with the presence of biofilm that complicates treatment options. Cardiologists often rely on conventional antibiotic therapy but with rising antimicrobial resistance rates, they sometimes offer little to no help in achieving stabilisation.
The strong properties of the Adtec SteriPlas cold plasma has been proven to offer complete stabilisation of the LVAD system by destroying all forms of multi-resistant bacteria present, even if they are protected within biofilm. This is due to the unique cold plasma properties that can travel through the gaps around and down the driveline to reach the site of biofilm. This area would normally have been difficult to treat with conventional therapies such as antimicrobial dressings as they are hard to reach, but the Adtec SteriPlas has shown to travel to these sites with ease due to its remarkable antibacterial gaseous properties.
To learn more about our strong collection of clinical evidence, please contact us at info@adtecplasma.com to learn more
Safe UV and Reactive Species levels only with the Adtec SteriPlas
As a leading medical device company, the crucial balance of safety and clinical efficacy are a top priority. We are always conducting tests to demonstrate the approved safety and reliability of the Adtec SteriPlas but to also learn new ways to improve the use of cold plasma in medicine.
Our clinical studies already validate the safety of the Adtec SteriPlas, including its low-level UV and reactive species produced from the cold plasma. These two components are imperative for the physical destruction of bacteria, however, too much of a good thing can also be relatively damaging like that observed with cold plasma jet/pens and battery powered cold plasma devices.
Measured across multiple distances, the UV light produced is well below the ICNIRP limit of 30 J/m2 and the reactive species produced are well below the occupational limits set by NIOSH and UK HSE. The Adtec SteriPlas continues to be one of the only cold plasma medical devices that puts patient and user safety first with no reported side effects and promising clinical efficacy from every treatment conducted.

For more information about the Adtec SteriPlas, send us an email at info@adtecplasma.com
SteriPlas' patented 6 electrode plasma system
Did you know our plasma chamber features a patented 6 electrode plasma torch system? This is where the cold plasma is generated before it is propelled with the gas flow over the treatment site. Our 60+ peer reviewed clinical studies have shown that accelerated healing in stalled and non-healing wounds/skin conditions can be achieved with no side effects reported.
Herz medizin 2021

We look forward to supporting Dr Heinrich Rotering’s presentation, “Avoiding sternal wire removal in early onset of sternal site infection - Two years experience with an optimized treatment concept using cold atmospheric plasma“ at the Annual scientific meeting of the German Society for Thoracic, Cardiac and Vascular Surgery. His presentation will discuss the strong benefits of using the Adtec SteriPlas for complicated sternal wound infections in cardiac patients.
The conference will be held virtually between 26-28 February: https://www.dgthg.de/de/jahrestagung