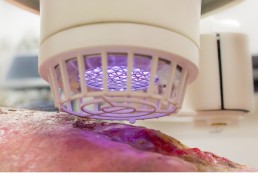
Size Does Matter!

Size does matter!
Yes, we do manufacture smaller handheld Cold Plasma devices that have antibacterial efficacy but we do not sell these in the medical field. A quick 2 minute treatment time is respective of being focused on the size of the plasma area created. Small and handheld plasma jet devices feature a 1mm2 treatment area which means antibacterial efficacy is achieved in a very small area for every 2 minutes applied. If the wound is 10cm2, we will leave you to do the maths on how long it will take to treat! This is why we do not sell handheld plasma jet pens as medical devices as they are limited to small and superficial wounds.
The SteriPlas creates a gigantic 12cm2 treatment area which is only possible through the size of the medical device which incorporates a series of crucial components such as the generator and control unit. This means the SteriPlas can cover large wounds and finish treatment in as quick as a single two minute session. Whilst the SteriPlas can treat acute and superficial wounds, it is designed to target deep and complicated wounds with #biofilm.
Deep sternal wounds is a classic example as documented in our clinical evidence.
Leading Cold Plasma medicine with the SteriPlas



The SteriPlas produces a repeatable, reliable, and highly efficacious Cold Plasma that has been proven in clinical trials and studies to destroy bacteria protected deep within biofilm.
Regardless of the resistance profile of bacteria, whether they are Gram-negative or Gram-positive, the physical mode of action of the SteriPlas Cold Plasma ensures the destruction of bacteria at the site of infection. This is particularly beneficial for difficult-to-treat or non-healing wounds such as diabetic foot ulcers, sternal wounds and LVAD infections. Where biofilm typically complicates the treatment options for these infections, the SteriPlas has been shown to fully heal patients even if they were not responding to antibiotic treatment to begin with thus saving limbs and lives.
All of this is achieved with the reputable safety of SteriPlas with no side effects reported.
How does the Adtec SteriPlas work so effectively?
Our patented cold plasma therapy utilises a physical mode of action approach delivered from an indirect microwave plasma source. Our cold argon plasma is an electrically charged and ionised gas that consists of charged particles, UV light and reactive oxygen and nitrogen species with the latter created as a by-product. They all work collectively to physically rupture the structure of bacteria even if they are protected within biofilm. Regardless of the type of bacteria, its resistance profile or whether they are Gram-positive or Gram-negative our cold plasma works quickly and effectively to destroy them. This has the leading advantage over conventional therapies such as antibiotics that rely on a chemical mode of action approach.
Although antibiotics have been a pivotal part of today’s medicine, they also contribute towards antimicrobial resistance rates particularly because bacteria can develop resistance such as those observed with diabetic foot ulcers that can often lead to amputations as a result.
However, as well as being fast-acting and effective, our cold plasma has shown that a primary or secondary resistance is unlikely to be developed due to its physical mode of action.

SteriPlas heals wounds quickly

Did you catch the leading Serbian media newspaper, Večernje Novosti, last week? Our SteriPlas was featured in the article, “Cold plasma heals wounds quickly” composed by Dr Zorić.
Dr Zoric has been using our cold plasma medical device to treat hard-to-heal and stalled wounds infected with biofilm. These problematic wounds have responded very well with our SteriPlas treatment and patients have been healed that were otherwise stalled by conventional therapies.
If you are within Serbia and interested to learn more about our medical device, contact our medical distributor Borf Health Care on info@borf.biz for more information.
Cold Plasma Diabetic Foot Ulcer Clinical Trial Published
Congratulations to Dr Cornelia Wiegand for her published clinical trial paper, “The efficacy of non-thermal gas plasma in the treatment of diabetic foot ulcers stalled by subclinical, biofilm-related wound infection”. This clinical trial paper features the use of our SteriPlas Cold Plasma medical device for the treatment of chronic DFUs infected with biofilm.
The parameters of this study were set much higher than a normal study, with there being a much higher standard of care given (3x per week) vs a typical outpatient setting of 1x weekly.
We wanted to show that even when chronic diabetic foot ulcers are subjected to more frequent outpatient visits with biopsies and swabs included (something not often collected during standard outpatient visits), SteriPlas Cold Plasma still prevails as the successful treatment method due to its physical mode of action and ability to kill all types of bacteria protected within biofilm.

Can two plasma medical devices have the same clinical efficacy?
We have been asked by Health Care Professionals to clarify , “What is the difference between the Adtec SteriPlas (Adtec Healthcare) and other cold plasma technologies such as Plasma Care® ?” or “Can two different plasma technologies have the same clinical efficacy… what’s the difference?”.
The answers to these questions are that there is a significant difference between plasma technologies, and it is therefore not possible to claim a similarity in clinical efficacy as the plasma treatments would be different.
Two devices do not produce the same plasma and it is the composition of the plasma that exerts the effects, beneficial and detrimental. For the importance of patient safety, Adtec Healthcare advises against any other plasma device claiming clinical efficacy from the clinical evidence of the CE approved SteriPlas gas plasma. Adtec’s clinical data should never be used to compare to any other plasma treatment which have not yet been clinically tested.
Atmospheric pressure Plasma has a myriad of potential medical applications from low energy plasmas used for wound healing to higher energy plasmas used to cut bones during an operation or for coagulation. The main differences in all plasma technologies include the different forms of plasmas electrode source designs, types of energy used (RF, DC and microwave) and gas used (argon, helium or nitrogen etc) versus air. The Adtec SteriPlas (MicroPlaSter) plasma technology is based on a microwave powered plasma jet utilising argon gas and the other technology is a DC powered Surface Micro Discharge (SMD) plasma source utilising air as carrier gas. The type of plasma generated corresponds directly to the type of treatment delivered to the patient. Each type of plasma delivers a specific type of treatment.
During the pre-clinical trials and studies, Max Planck had conducted a study illustrating the noticeable differences of our argon gas microwave plasma treatment vs the surface micro-discharge (SMD) ‘Air plasma’ treatment developed at MPI. In this study, clear distinctions could be observed such as argon gas plasma treatment included predictable, safe and low dosages of reactive species whereas air-based plasmas would deliver significantly higher concentrations (over 37 times more than argon microwave plasma). Air Plasma has much higher levels of NOx and Ozone than argon gas plasma and needs to be tested not only clinically but also safety for the operator. It is essential to verify the product safety and clinical safety of these higher levels of NOx. The air-based plasmas are also generally dependent on the surrounding environment; temperature and humidity will affect the way the plasmas are generated and therefore difficult to deliver the same treatment each time the plasma is generated.

Adtec Plasma Technology has been at the forefront for the development of plasma products for over 30 years, proudly placing us as one of the leaders in the semiconductor and RF plasma market. Adtec has also developed other plasma technologies (gas and air) for remote plasma, gas abatement and surface treatment applications.
In 2002, we designed our first cold plasma technology showing painless effect on contact with human skin. This revolutionised the way how we are used to dealing with plasmas – typically sought as too hot to touch but now redesigned to be colder and harmless on contact with skin. In 2004, our research results were presented at a plasma conference showing distinctive microbial load reduction was possible when bacteria were exposed to our gas plasma.
Later in 2004, Adtec introduced this plasma technology to the Max Planck Institute for Extraterrestrial Physics leading to a collaboration in plasma medicine. The Adtec plasma source was adapted to a wide area plasma source in collaboration with MPI. This plasma source of the medical device is a shared patent with Adtec and MPI (as seen in the image below). This plasma source is one of the critical components used in the SteriPlas and MicroPlaSter. Adtec solely and exclusively designed and developed the medical device prototypes and products including customized components, electrical, mechanical and software program satisfying the strict standards of European medical device regulations.


We are grateful to the plasma medicine team at Max Planck for having an interest in our plasma technology system, for carrying out extensive scientific research and for managing the clinical trials using the Adtec MicroPlaSter. These clinical trials placed our medical device as the first worldwide to be used in clinical trials on wounds, paving a new treatment programme that would later be adopted by other companies with an interest to develop gas plasma medical devices. The extensive research and clinical testing assure us of the safety of the product and the technology.
The new European MDR regulations coming into force next year also require all companies to produce proper clinical evidence to support their product claims. We do emphasize the importance of proper pre-clinical and clinical testing of all types of plasma technologies in order to be assured of the product and clinical safety. Adtec Healthcare do encourage and support plasma companies in this industry as our goal is to make gas plasma a recognised treatment option for patients with wounds, surgical site infections and dermatological conditions.