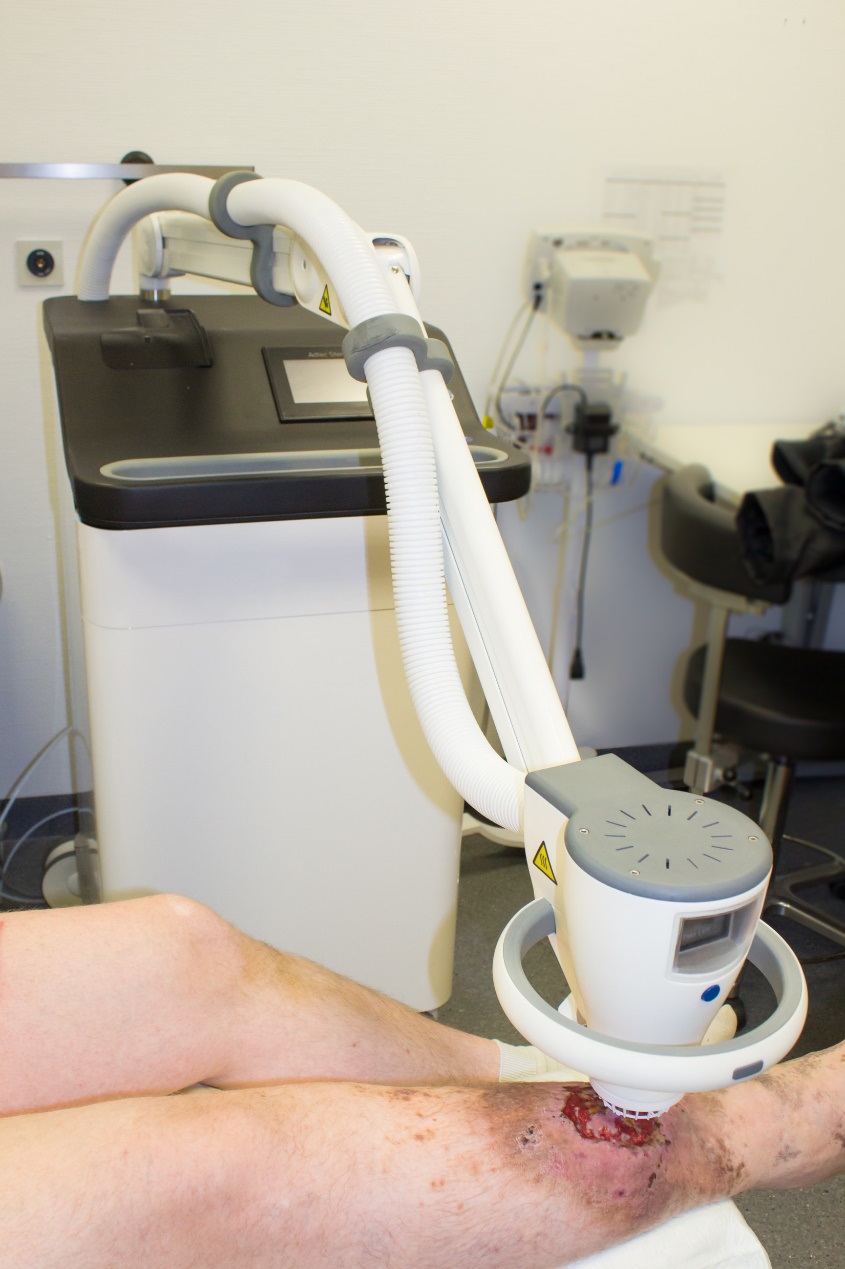
Casopis Lecba ran-page-003
Did you see the Adtec SteriPlas featured in the Czech Society for Wound Healing magazine?
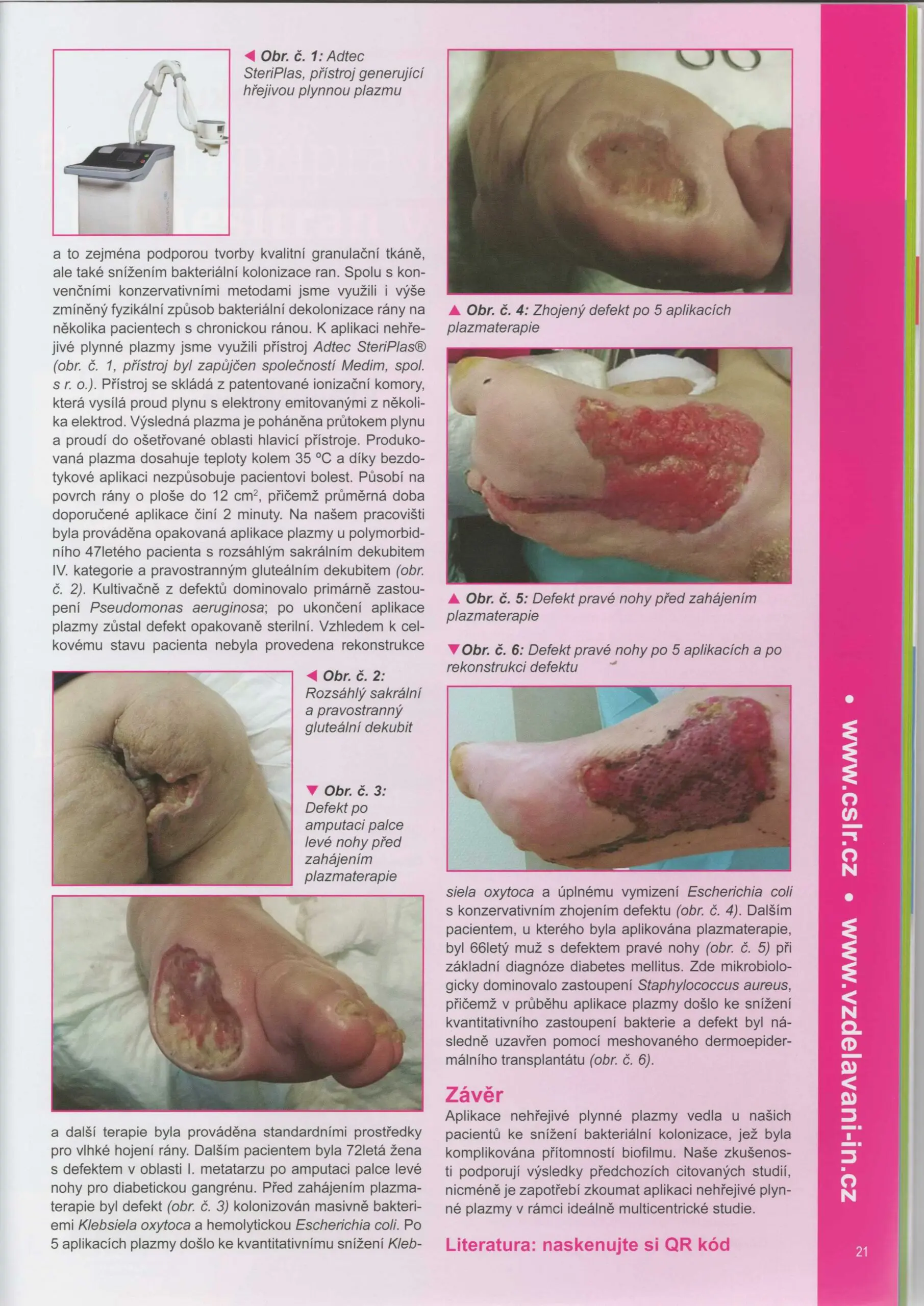
Dr Hokynková from the Department of Burns and Plastic Surgery at Brno Hospital in Czech Republic had published a case study in the magazine to document the efficacy of the Adtec SteriPlas after having tested this on a variety of wounds including pressure ulcers and diabetic foot ulcers.
The article validates the SteriPlas’ ability to significantly reduce microbial load within the wound which allows the body to regain control and accelerate healing of stalled wounds. This includes wounds that are already contaminated with biofilm and listed for amputations. A change from the conventional therapies already existing on the market, the Adtec SteriPlas has proven to reverse further deterioration of complicated wounds and allow full healing and stability to be achieved.
For a translation of the article or to learn more about our efficacy and treatment options, please email us at info@adtecplasma.com


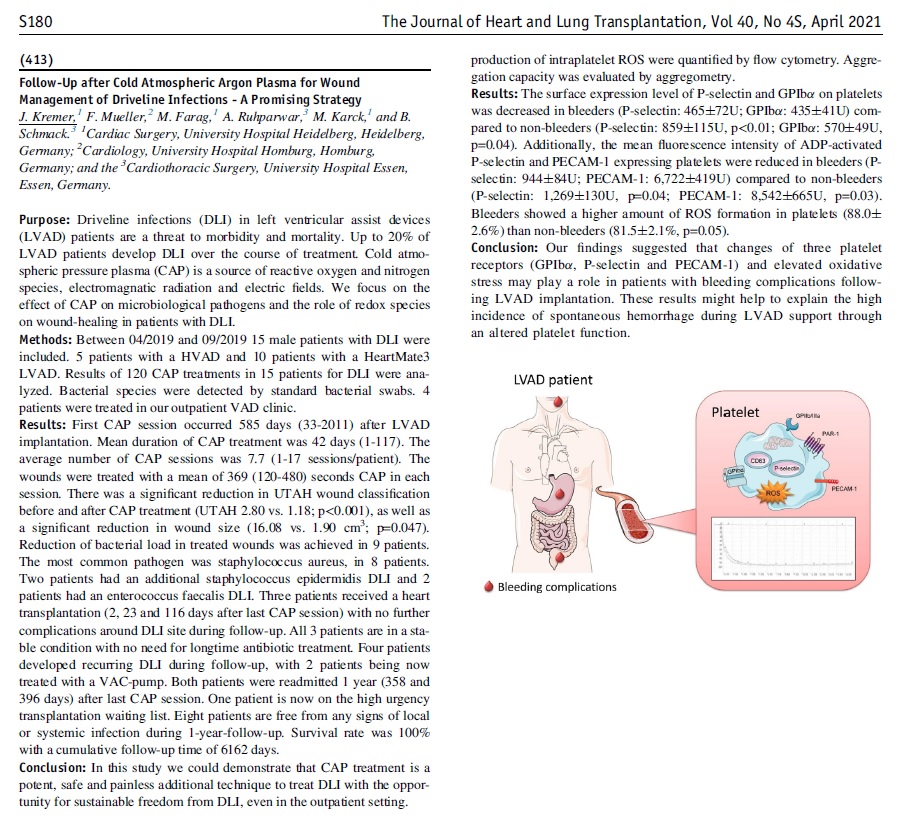
LVAD - Heidelberg publication 2021
Congratulations to Dr Kremer and the Cardiology Team at The University Hospital Heidelberg for their new publication, “Follow-Up after Cold Atmospheric Argon Plasma for Wound Management of Driveline Infections - A Promising Strategy”. This publication features the use of our Adtec SteriPlas medical device for the treatment of driveline infections (DLI). The results demonstrate how the safety, reliability and painless treatment from the Adtec SteriPlas can significantly reduce the wound and infection around the DLI, with the opportunity for sustainable freedom from DLI.
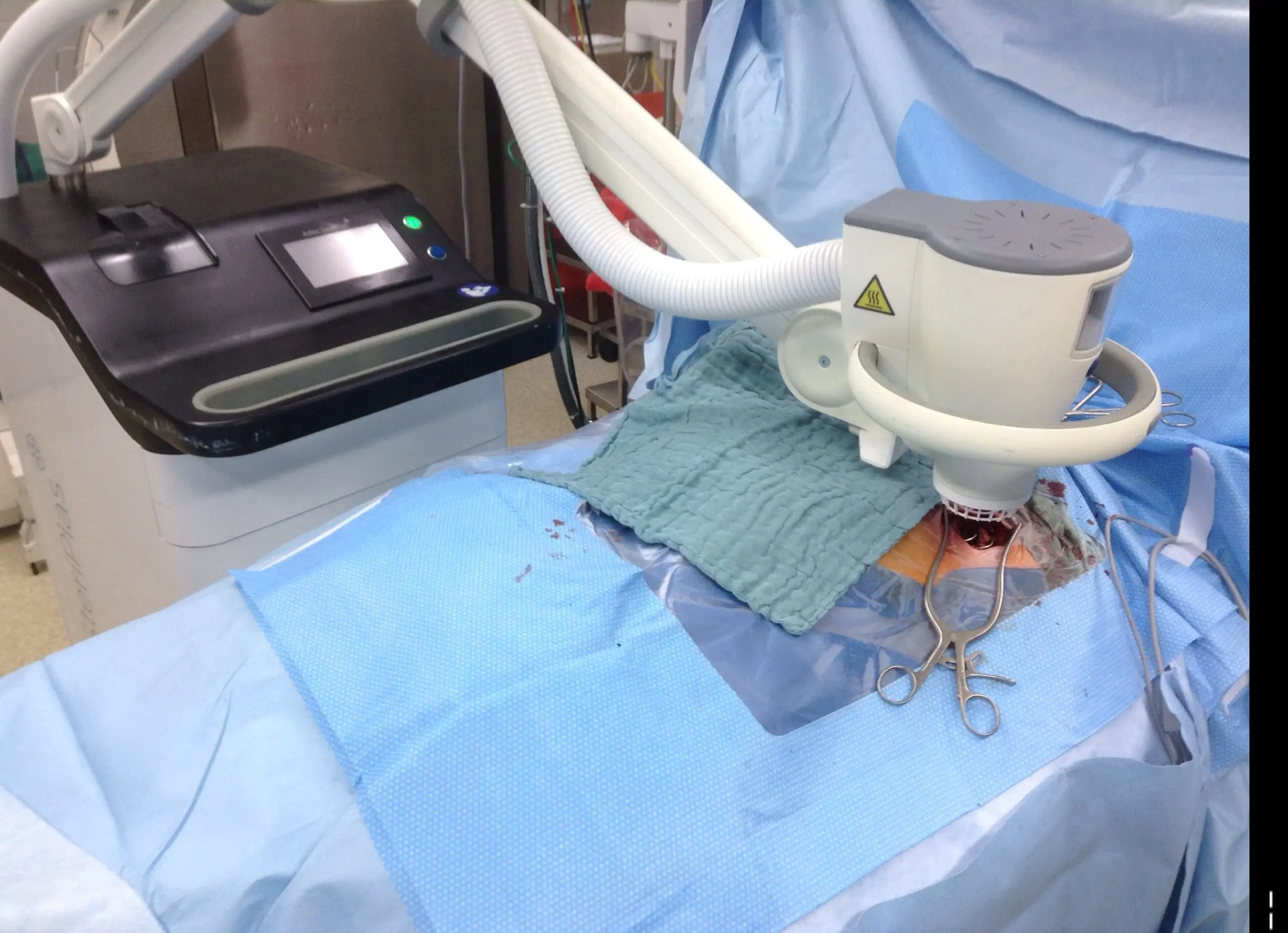
Deep Sternum Surgical Site Infections (DSSIs)
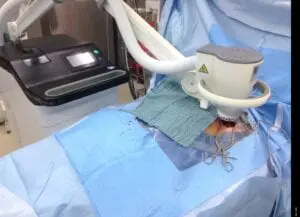
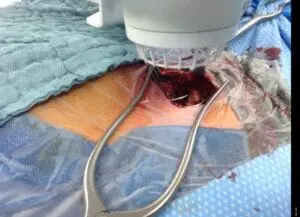
Deep sternal surgical site infections (DSSIs) are a still a severe complication after cardiac surgery. The mortality estimated range is between 15%-40% of all cases. In addition to the significantly extended hospitalization, there is a significant cost burden with a calculated cost of € 36,000 for a single DSSI case.
The use of the Adtec SteriPlas for these complicated conditions has been actively documented as better managing the chronic infections, reducing hospitalization times and cost but more importantly it has shown to decrease the mortality rate. Our medical device has been praised as a tissue and life saving medical device.
Herz medizin 2021

We look forward to supporting Dr Heinrich Rotering’s presentation, “Avoiding sternal wire removal in early onset of sternal site infection - Two years experience with an optimized treatment concept using cold atmospheric plasma“ at the Annual scientific meeting of the German Society for Thoracic, Cardiac and Vascular Surgery. His presentation will discuss the strong benefits of using the Adtec SteriPlas for complicated sternal wound infections in cardiac patients.
The conference will be held virtually between 26-28 February: https://www.dgthg.de/de/jahrestagung
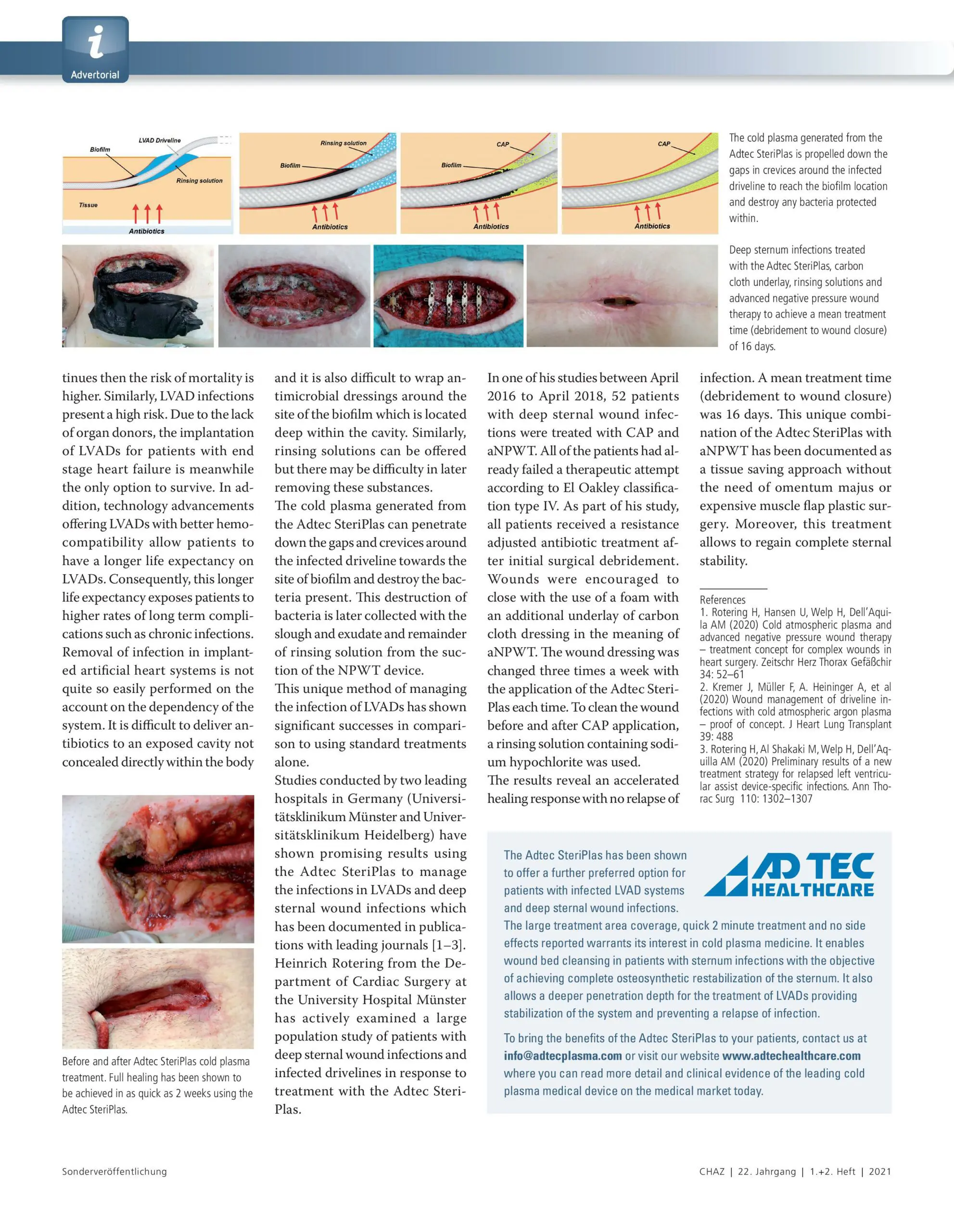
Adtec SteriPlas for the treatment of deep sternal wound infections and infected drivelines
The Adtec SteriPlas has already been well documented for the treatment of deep sternal wound infections and infected drivelines. Separate studies from two leading hospitals in Germany (University Hospital Munster and University Hospital Heidelberg) have recorded the strong efficacy of our cold plasma treatment on these problematic conditions. Due to the original difficulty of managing these chronic infections using standard therapies along with the high rate of morbidity and mortality, it is no wonder why the Adtec SteriPlas has been chosen as the preferred treatment method.
You can read more about its story in the latest addition of the Chirurgische Allgemeine magazine: eSD_AdTecPlasma


SteriPlas cold plasma successful at killing multi-resistant bacteria
Our animation video shows how bacteria are easily destroyed using our patented cold plasma technology. The components of cold plasma work collectively to target bacteria and physically rupture the structure, allowing micropores to be created which gains access to the microbial DNA to be destroyed. This effectively kills bacteria, even if they are protected within biofilm.
Learn more about our clinical evidence by visiting our webpage www.adtechealthcare.com or send us an email at info@adtecplasma.com
Click the link below to access the animation video:
Animation video to show how the SteriPlas can successfully kill multi-resistant bacteria
New SteriPlas version and change in device Classification
Due to our proven clinical success in treating non-healing and challenging wounds, we are proud to announce the upgrade of our medical device classification to Class IIb for the Adtec SteriPlas .
This change in medical device classification also supports its continued use for treating chronic and deep wounds and surgical site infections and for treating dermatological conditions.
We also announce the product launch of a new version of our SteriPlas Version 2 model in our Cold Plasma medical device family delivering the same performance. We are very proud of the safety of our devices with no incidents or adverse events reported in 15 years of use.
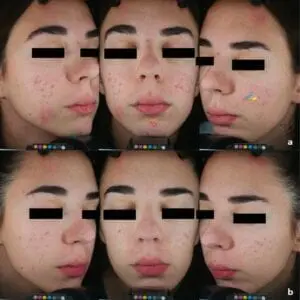
SteriPlas treatment for Acne Vulgaris
We are excited to announce the recent publication, “Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris” which features our Adtec SteriPlas cold plasma for the treatment of acne.
The paper created by Dr Mariachiara Arisi from the Department of Dermatology at the University of Brescia, Italy demonstrates the antibacterial efficacy of our patented cold plasma for the treatment of acne vulgaris patients. It indicates the significant reduction of acne skin lesions in treated patients who were previously unsuccessful treated with topical drugs. Unlike topical drugs, the Adtec SteriPlas demonstrated a safe, effective, and well-tolerated treatment option with no side effects for the treatment of acne patients.
No adverse effects or skin reactions were reported either during the treatment nor at 3-months follow-up. Treatment was completely painless and well tolerated. Patients did not report itching or burning sensation during plasma application and in the following days.
The full paper can be found here: https://www.sciencedirect.com/science/article/pii/S2212816620300172
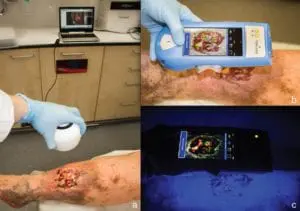
Adtec Healthcare celebrates yet another RCT publication

Adtec Healthcare celebrates another prospective, randomized and placebo-controlled trial (RCT) publication featuring our cold plasma medical device for the treatment of chronic wounds. The clinical trial conducted at the Essen University Medical Center in Germany and led by Professor Joachim Dissemond discusses the strong benefit of using the Adtec SteriPlas to treat chronic wounds. This is our first RCT measuring wound healing rates with respect to overall wound size using cold plasma.
The study consisting of three groups (Group 1 - plasma 1x/week, Group 2- plasma 3x/week and Group 3 - placebo 1x/week) shows the significant wound size reduction when using cold plasma vs placebo. Not surprisingly, Groups 1 and 2 led an astonishing 63.0% and 46.8% reduction in wound size for chronic wound patients, whereas Group 3 (placebo-treated) patients had increased in wound size by 17.5% larger.
The full publication can be viewed here: https://onlinelibrary.wiley.com/doi/epdf/10.1111/ddg.14294
The rich data of the RCT further demonstrates why our cold plasma medical device, the Adtec SteriPlas, continues to lead the way for cold plasma medicine by accelerating healing in non-healing and problematic wounds with the strong advantage of no side effects. The use of the Adtec SteriPlas is simple – a quick supplement to your standard treatment program for your patients.
Contact us at info@adtecplasma.com to learn more about our medical device and how to obtain this in your clinic.
Leading cold plasma medical device
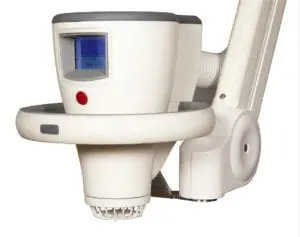
The Adtec SteriPlas is the leading cold plasma medical device used to manage the infection in chronic wounds, surgical site infections and to treat dermatological conditions such as acne and actinic keratoses.
With our wide clinical trials and publications bibliography, we are proud to state its strong antibacterial efficacy regardless of the type of bacteria or its resistance profile. There have been no side effects reported with the use of our medical device since it is safe, painless to touch and an effective method of treating patients from conventional therapies.