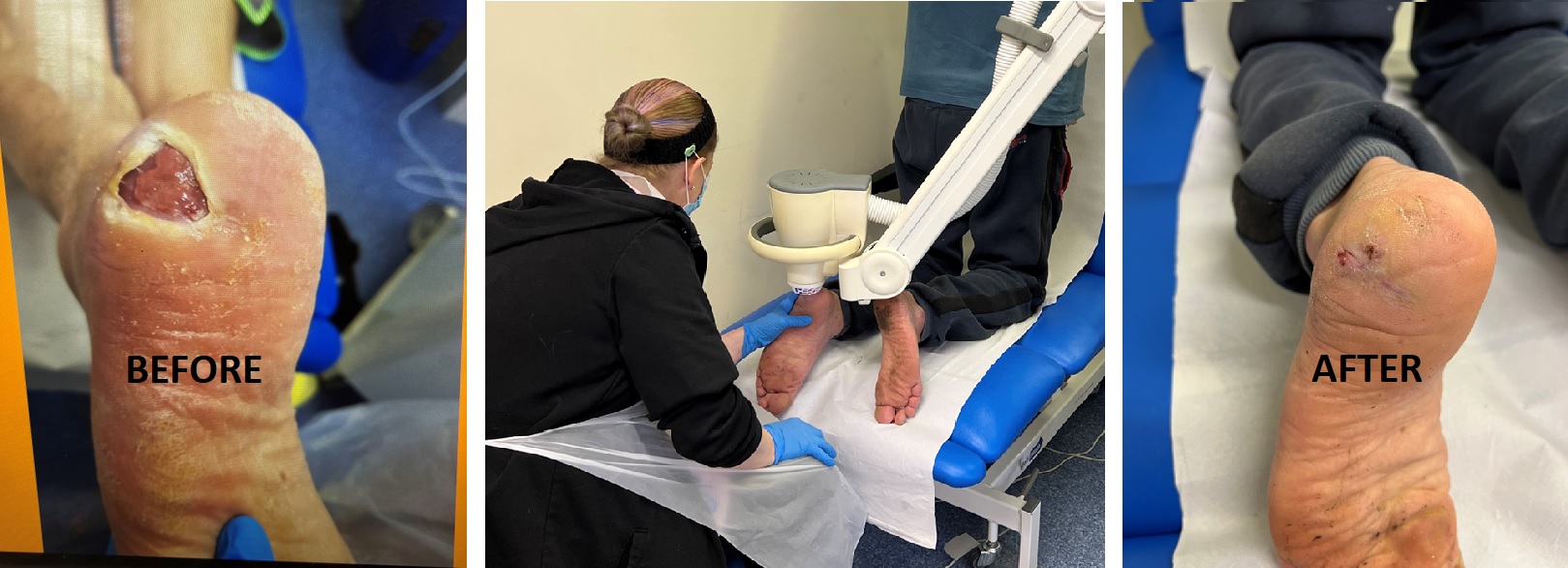
The Power of Going Large

The SteriPlas boasts a wide clinical bibliography of clinical trials and publications, documenting the strong clinical efficacy coupled with no side effects reported. This benefits to patients suffering from non-healing wounds infected with biofilm whereby quick 2 minute treatment sessions are able to destroy all bacteria present and heal patients in as quick as 1 week.
To make such a statement as the above requires strong, reputable, and quality-driven results which we are thankful to have. But to collect these results and optimise the technical build of the SteriPlas has taken many years. From our first ever clinical trial on wounds approximately two decades ago to today, the SteriPlas has had its fair share of changes.
Whilst there have been multiple beneficial changes, the overall size of the SteriPlas has remained the same for a crucial reason. The Steriplas is the size it is to produce the optimum plasma for clinical efficacy using microwave argon plasma. Whilst Cold Plasma can still be generated with various technologies, it cannot be done in the same dense and rich manner delivering antibiofilm results. Crucial components to ensure the consistency and reliability may be lost. Most cold plasmas will have antibacterial efficacy against planktonic strains in in-vitro non-competing environments, but chronic wounds are a complex topography of biofilm, exudate, slough, living and necrotic tissue.
Adtec Plasma Technology has developed many Cold Plasma devices since its existence from small pens, portable shoulder strap machines to large medical devices. Our legacy device, the SteriPlas, remains the clinical leader in Cold Plasma medicine for wounds, surgical site infections and medical dermatology conditions.
Seasons Greetings!

Seasons Greetings!
May the new year be even more productive and cheerful than the previous one. Thank you to all our customers, distributors, suppliers and to everyone who has supported us. We would not be where we are today if not for you. Wishing you all the best this season and always.
Adtec to present at the Advanced Wound Care Summit EU

We’ve been invited to speak at the Advanced Wound Care Summit EU conference in London this week. Our Senior Business Development Manager, Jeiram, will be part of the Executive Speakers Line Up at the opening session “Panel Prioritising Clinical Data & Health Economic Evidence” at 8.55am on Tuesday 28th November.
Adtec Healthcare strives for strong and reputable clinical evidence. Over the years of our existence, we have collected a total of 80+ peer reviewed clinical studies on the SteriPlas Premium Cold Plasma which has resulted in quality Health Economics data for our medical device.
We understand the absolute importance of Health Economics data to back the claims of medical devices. With our clinical evidence, we can present the promising results of the SteriPlas for significantly accelerating wounds stalled with biofilm and reducing bed occupancy, antibiotics usage and the financial burden posed on hospitals.
Our Presentation video from the LSI Emerging Medtech Conference
Last month our CEO had presented the strong antibacterial benefits, clinical evidence and our business plan for our Premium Cold Plasma at the Life Science Intelligence (LSI) Emerging Medtech Conference.
After much positive interactions since the LSI which has led to promising meetings, we share with you that presentation : https://fb.watch/nWbLL1E_8d/
Day 2 of EWMA
It's day 2 at EWMA 2023 and we're having a great time meeting with new and existing customers. If you want to learn about the most effective Cold Plasma medical device for woundccare with the most clinical trials, strongest efficacy and no side effects, please visit our booth at no. 116 to see a live demonstration of the SteriPlas in action.


How does the Adtec SteriPlas work so effectively?
Our patented cold plasma therapy utilises a physical mode of action approach delivered from an indirect microwave plasma source. Our cold argon plasma is an electrically charged and ionised gas that consists of charged particles, UV light and reactive oxygen and nitrogen species with the latter created as a by-product. They all work collectively to physically rupture the structure of bacteria even if they are protected within biofilm. Regardless of the type of bacteria, its resistance profile or whether they are Gram-positive or Gram-negative our cold plasma works quickly and effectively to destroy them. This has the leading advantage over conventional therapies such as antibiotics that rely on a chemical mode of action approach.
Although antibiotics have been a pivotal part of today’s medicine, they also contribute towards antimicrobial resistance rates particularly because bacteria can develop resistance such as those observed with diabetic foot ulcers that can often lead to amputations as a result.
However, as well as being fast-acting and effective, our cold plasma has shown that a primary or secondary resistance is unlikely to be developed due to its physical mode of action.
Adtec at the Diabetic Foot Study Group conference in Bratislava
Don't forget to visit us at the Diabetic Foot Study Group (DFSG) conference this weekend. We're having a great time welcoming you to our booth. Our SteriPlas is here ready to give you a live demonstration on how easy it is to use.




Our deepest condolences to the Royal Family
The Safety and Reliability of our Cold Plasma
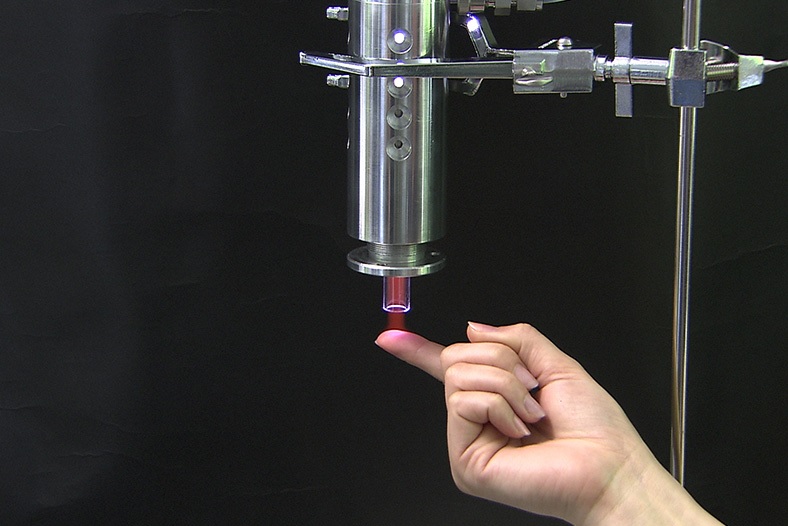
Is our cold plasma safe?
Yes!
As you can see from the photo, our Adtec technician demonstrates touching the cold plasma jet.
An extensive list of practical tests and experiments were conducted during our first stage, pre clinical studies and ongoing post-market studies. This allowed us to carefully develop a cold plasma medical device that could bring all the antibacterial benefits but at the same time remain painless and contact-free so that it could be well tolerated in patient treatments. We were after all the first company worldwide to introduce cold plasma treatment on wounds demonstrated in our clinical trials.
As cold plasma can be generated through a variety of ways, they are also dependent on the plasma source design and surrounding environment. The components of plasma include reactive oxygen and nitrogen species and UV that need to be controlled to ensure they are within safe levels for medical use. Some plasma devices may also deliver fluctuating inconsistent cold plasma output as they are sensitive to changes in humidity and temperature in the treatment room. This may often result in undesirable side effects.
Because the Adtec SteriPlas is a low energy, microwave-powered, cold atmospheric argon plasma medical device this means its safety remains the main priority in line with delivering repeatable and successful results. It is also the reason why we are proud to state our medical device has NO side effects associated to it.
Our patented 6 electrode Cold Plasma Torch

The Adtec SteriPlas features a patented 6 electrode cold plasma torch. It’s proven efficacy for the destruction of all forms of bacteria including multi-resistant bugs makes it an alternative to antibiotic therapy for the treatment of wounds, surgical site infections and medical dermatology skin conditions. This is due to the unique physical mode of action delivered with our cold plasma as opposed to the chemical mode of action demonstrated with antibiotics. Broader studies have shown that antimicrobial resistance is unlikely to be generated with cold plasma treatment because of this.
A huge collection of our peer reviewed publications documents the clinical efficacy and safety of our medical device across a broad range of conditions including diabetic foot ulcers, left ventricular assist device (LVAD) infections, deep sternal wound infections, actinic keratoses and acne.
To learn more about our medical device or to obtain one in your clinic practice, please contact us at info@adtecplasma.com