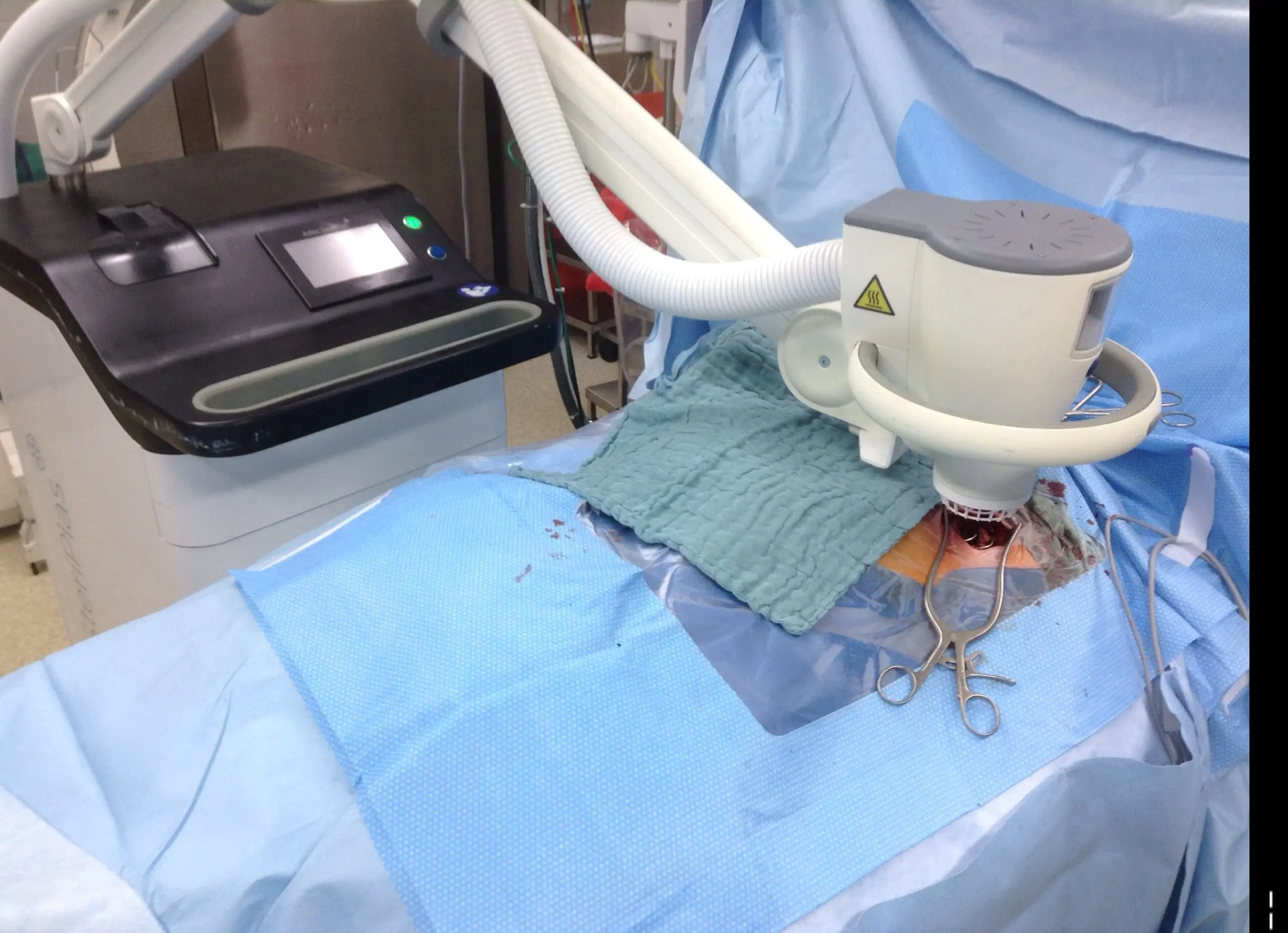
The ISHLT discusses the Cold Plasma Project
We’re ecstatic to see our Adtec SteriPlas continue to raise awareness in the LVAD community. A great interview between the ISHLT and Mr Thomas Schlöglhofer from the Medical University of Vienna discussing the benefits of our cold atmospheric plasma medical device for the treatment of LVAD Infections and their broad study being conducted.
Thomas has been an avid user of our medical device for some time now collecting remarkable results with his LVAD patients, documenting the strong benefits of our medical device including the significant reduction of infection rates coupled with full healing, reduction of readmittance and the stabilisation of LVAD systems all with the added benefit of no side effects.
We look forward to the completion of this study and the publishing of the data which will be discussed at the ISHLT 2023 conference next year. We will also be exhibiting at this conference and excited to meet more people from the LVAD community.
https://youtu.be/6-L0sgXLx9o
Characterisation of a Cold Atmospheric Pressure Plasma Torch for Medical Applications
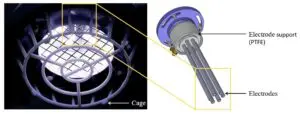
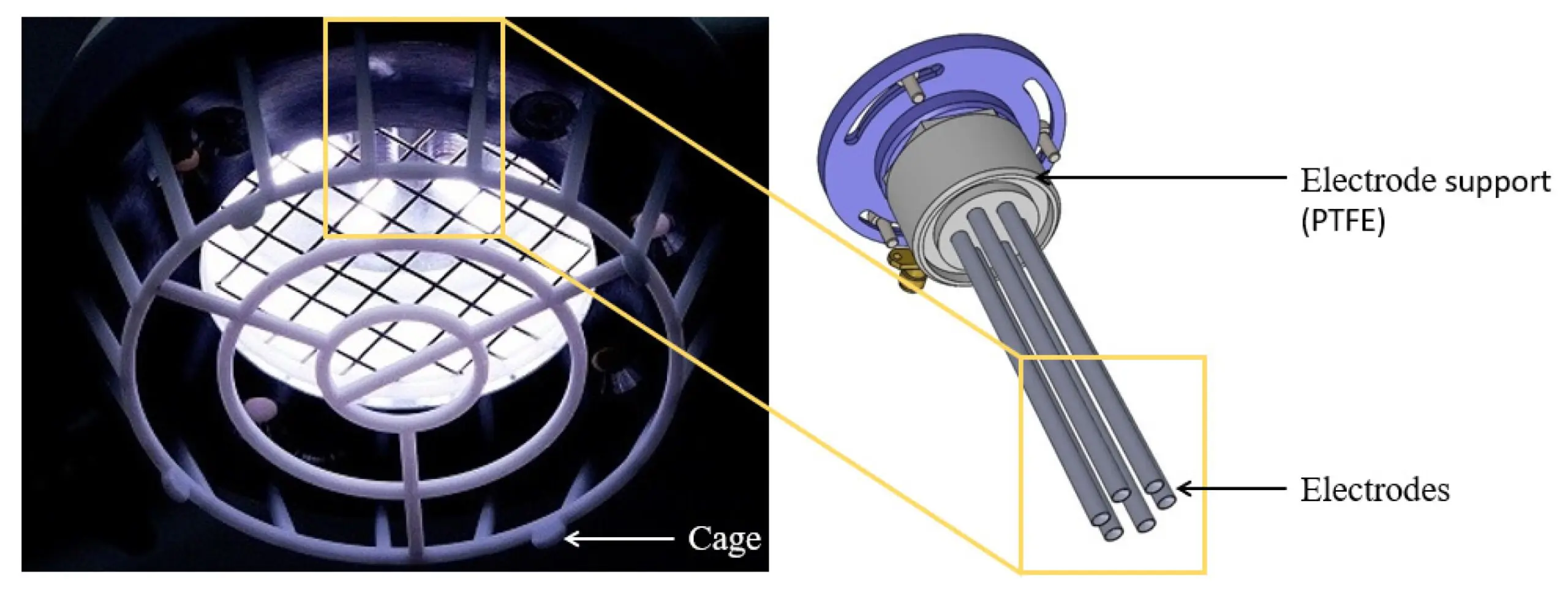
We congratulate Dr Adam Bennett on his most recent publication, “Characterisation of a Cold Atmospheric Pressure Plasma Torch for Medical Applications: Demonstration of Device Safety”.
The safety and effectiveness of plasma devices are of crucial importance, especially for applications where the plasma is discharged near humans. This study presents the novel design and characterisation of a Cold Atmospheric Plasma torch (SteriPlas), which is used in medical applications. The study shows the characterisation methodology that must be undertaken to show that a plasma device is safe, especially when used in an application on human skin. The emission spectrum discharged from the plasma torch is shown. The UV emitted is measured and the effective irradiance is calculated. The effective irradiance enables the determination of the maximum UV exposure limits, which in this application are shown to be over two hours; however, in some applications may be only seconds. NOx and ozone emissions are also recorded. The NOx levels in this application are shown to be orders of magnitude lower than their safety limits and the ozone emissions are also shown to be safe; however, in some plasma technologies the NOx and ozone levels are orders of magnitude higher than the safe levels.
This paper concludes with a discussion of how safety limits vary in different regions around the world and proposes an international standard. It documents the safety of our medical device which further reiterates one of our main strengths where no side effects have been reported.
Access to the full paper can be found here: https://www.mdpi.com/2076-3417/11/24/11864
The first cold plasma medical device to treat Scleroderma

We congratulate Dr Stephanie Ardnt and Prof. Sigrid Karrer from the University Hospital Regensburg for their recent publication, “The Anti-Fibrotic Effect of Cold Atmospheric Plasma on Localized Scleroderma In Vitro and In Vivo”.
This publication features the use of our Adtec SteriPlas and shows strong efficacy for the treatment of Scleroderma, which up to now has not yet been evaluated by CAP. The extensive study documents significantly reduced dermal thickness and collagen deposition as well as a decrease in both alpha smooth muscle actin-positive myofibroblasts and CD68-positive macrophages in the affected skin in comparison to untreated fibrotic tissue. This study provides the first evidence for the successful use of CAP for treating LS and may be the basis for clinical trials including patients with LS.
https://doi.org/10.3390/biomedicines9111545
Safe UV and Reactive Species levels only with the Adtec SteriPlas
As a leading medical device company, the crucial balance of safety and clinical efficacy are a top priority. We are always conducting tests to demonstrate the approved safety and reliability of the Adtec SteriPlas but to also learn new ways to improve the use of cold plasma in medicine.
Our clinical studies already validate the safety of the Adtec SteriPlas, including its low-level UV and reactive species produced from the cold plasma. These two components are imperative for the physical destruction of bacteria, however, too much of a good thing can also be relatively damaging like that observed with cold plasma jet/pens and battery powered cold plasma devices.
Measured across multiple distances, the UV light produced is well below the ICNIRP limit of 30 J/m2 and the reactive species produced are well below the occupational limits set by NIOSH and UK HSE. The Adtec SteriPlas continues to be one of the only cold plasma medical devices that puts patient and user safety first with no reported side effects and promising clinical efficacy from every treatment conducted.

For more information about the Adtec SteriPlas, send us an email at info@adtecplasma.com
Casopis Lecba ran-page-003
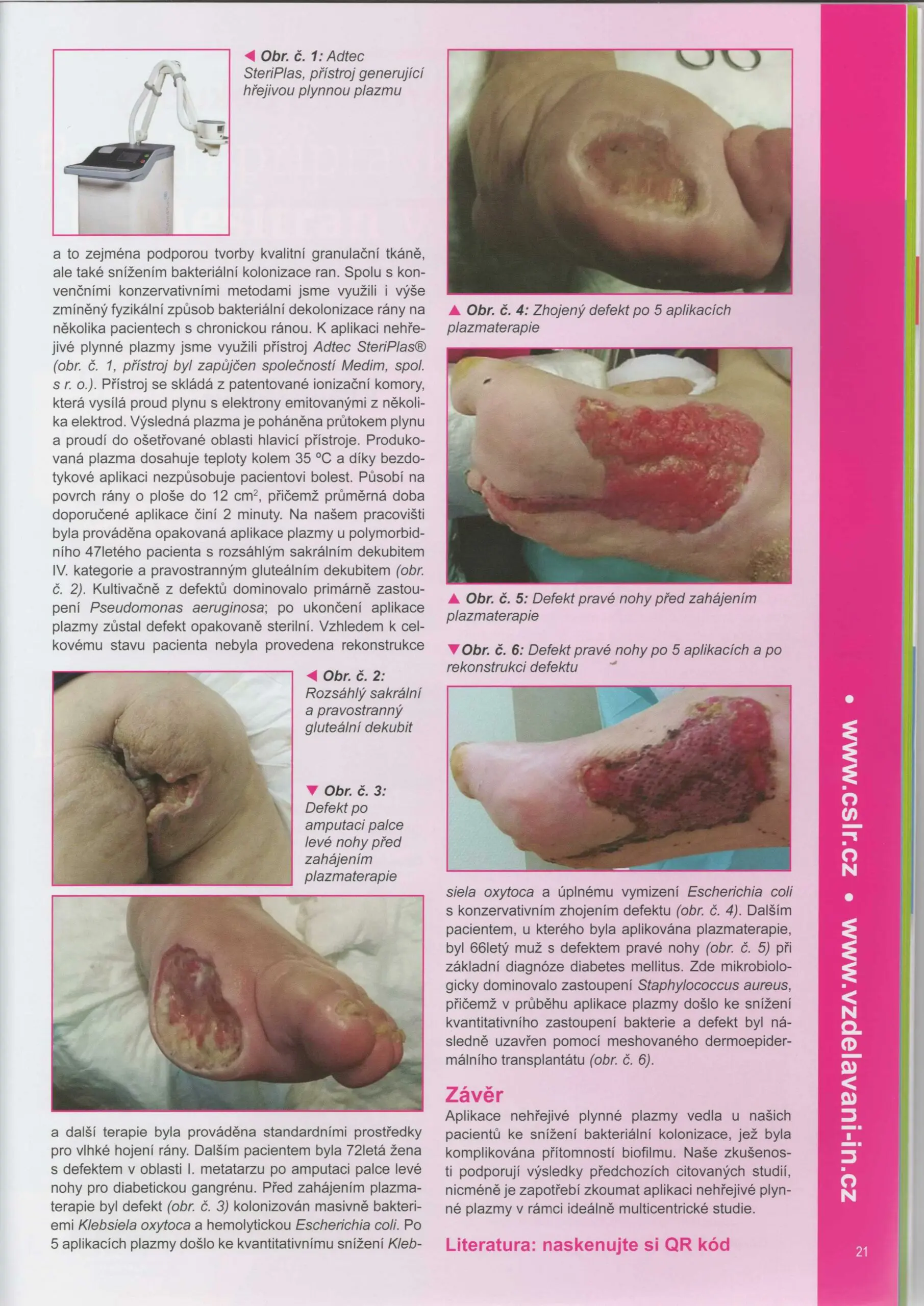
Did you see the Adtec SteriPlas featured in the Czech Society for Wound Healing magazine?
Dr Hokynková from the Department of Burns and Plastic Surgery at Brno Hospital in Czech Republic had published a case study in the magazine to document the efficacy of the Adtec SteriPlas after having tested this on a variety of wounds including pressure ulcers and diabetic foot ulcers.
The article validates the SteriPlas’ ability to significantly reduce microbial load within the wound which allows the body to regain control and accelerate healing of stalled wounds. This includes wounds that are already contaminated with biofilm and listed for amputations. A change from the conventional therapies already existing on the market, the Adtec SteriPlas has proven to reverse further deterioration of complicated wounds and allow full healing and stability to be achieved.
For a translation of the article or to learn more about our efficacy and treatment options, please email us at info@adtecplasma.com


LVAD - Heidelberg publication 2021
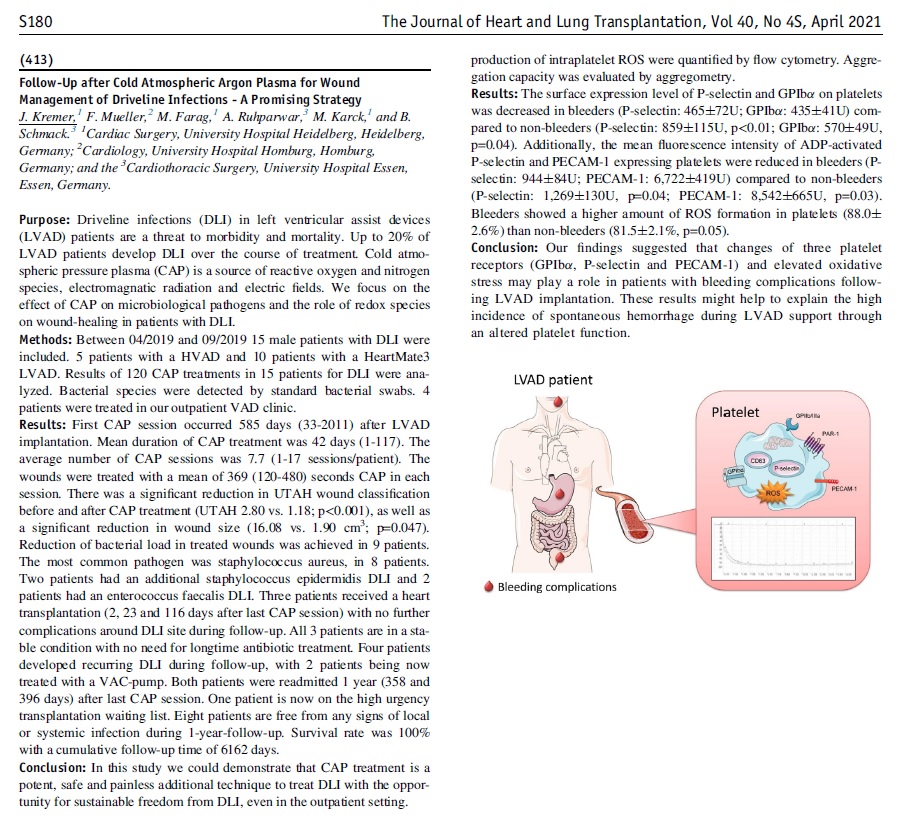
Congratulations to Dr Kremer and the Cardiology Team at The University Hospital Heidelberg for their new publication, “Follow-Up after Cold Atmospheric Argon Plasma for Wound Management of Driveline Infections - A Promising Strategy”. This publication features the use of our Adtec SteriPlas medical device for the treatment of driveline infections (DLI). The results demonstrate how the safety, reliability and painless treatment from the Adtec SteriPlas can significantly reduce the wound and infection around the DLI, with the opportunity for sustainable freedom from DLI.
The SteriPlas sets foot in the U.S.A.

We are excited to share news of our collaboration with The Southern Illinois University in United States of America. Our Adtec SteriPlas will be used as part of their study to examine the effect of cold plasma on a variety of infection burn model cases in a research laboratory setting. This study aims to help tackle the outbreaks of antimicrobial resistant microorganisms that continues to be an issue for burns cases. We look forward to providing more information and the results as the study progresses.
Deep Sternum Surgical Site Infections (DSSIs)
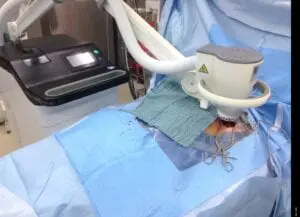
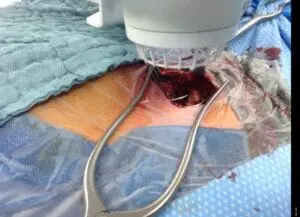
Deep sternal surgical site infections (DSSIs) are a still a severe complication after cardiac surgery. The mortality estimated range is between 15%-40% of all cases. In addition to the significantly extended hospitalization, there is a significant cost burden with a calculated cost of € 36,000 for a single DSSI case.
The use of the Adtec SteriPlas for these complicated conditions has been actively documented as better managing the chronic infections, reducing hospitalization times and cost but more importantly it has shown to decrease the mortality rate. Our medical device has been praised as a tissue and life saving medical device.
Patient and user safety is priority with the Adtec SteriPlas
As a medical device company, we always strive to ensure the priority of patient and user safety. In comparison to battery-powered and smaller cold plasma devices which may produce unreliable and damaging levels of ozone and NOx, the Adtec SteriPlas has been carefully designed as a safe and well-balanced cold plasma medical device. It has been engineered to deliver predictable levels of cold plasma to ensure the continuity of no side effects reported, putting safety and efficacy at the top priority.

New SteriPlas version and change in device Classification
Due to our proven clinical success in treating non-healing and challenging wounds, we are proud to announce the upgrade of our medical device classification to Class IIb for the Adtec SteriPlas .
This change in medical device classification also supports its continued use for treating chronic and deep wounds and surgical site infections and for treating dermatological conditions.
We also announce the product launch of a new version of our SteriPlas Version 2 model in our Cold Plasma medical device family delivering the same performance. We are very proud of the safety of our devices with no incidents or adverse events reported in 15 years of use.