Safe UV and Reactive Species levels only with the Adtec SteriPlas
As a leading medical device company, the crucial balance of safety and clinical efficacy are a top priority. We are always conducting tests to demonstrate the approved safety and reliability of the Adtec SteriPlas but to also learn new ways to improve the use of cold plasma in medicine.
Our clinical studies already validate the safety of the Adtec SteriPlas, including its low-level UV and reactive species produced from the cold plasma. These two components are imperative for the physical destruction of bacteria, however, too much of a good thing can also be relatively damaging like that observed with cold plasma jet/pens and battery powered cold plasma devices.
Measured across multiple distances, the UV light produced is well below the ICNIRP limit of 30 J/m2 and the reactive species produced are well below the occupational limits set by NIOSH and UK HSE. The Adtec SteriPlas continues to be one of the only cold plasma medical devices that puts patient and user safety first with no reported side effects and promising clinical efficacy from every treatment conducted.

For more information about the Adtec SteriPlas, send us an email at info@adtecplasma.com
Adtec Healthcare prepares for exhibition in July

We are very excited to be exhibiting at the Malvern Diabetic Foot Conference between 7-9th July. This will be our first physical exhibition participation in over a year! Be sure to visit us and see our cold plasma medical device live in action, perfect for the treatment of chronic DFUs infected with biofilm.
E-poster at the EADV 2021 Conference

We look forward to supporting Fiona Koch’s e-poster that will be live during the EADV 2021 Spring Symposium tomorrow. Tune in to their website to get full access to her poster, “Efficacy of cold atmospheric plasma versus diclophenac 3% gel in patients with actinic keratoses: a prospective, randomized and rater-blinded study (ACTICAP)”.
To learn more about the Adtec SteriPlas’ strong clinical efficacy for the treatment of actinic keratoses, contact us at info@adtecplasma.com
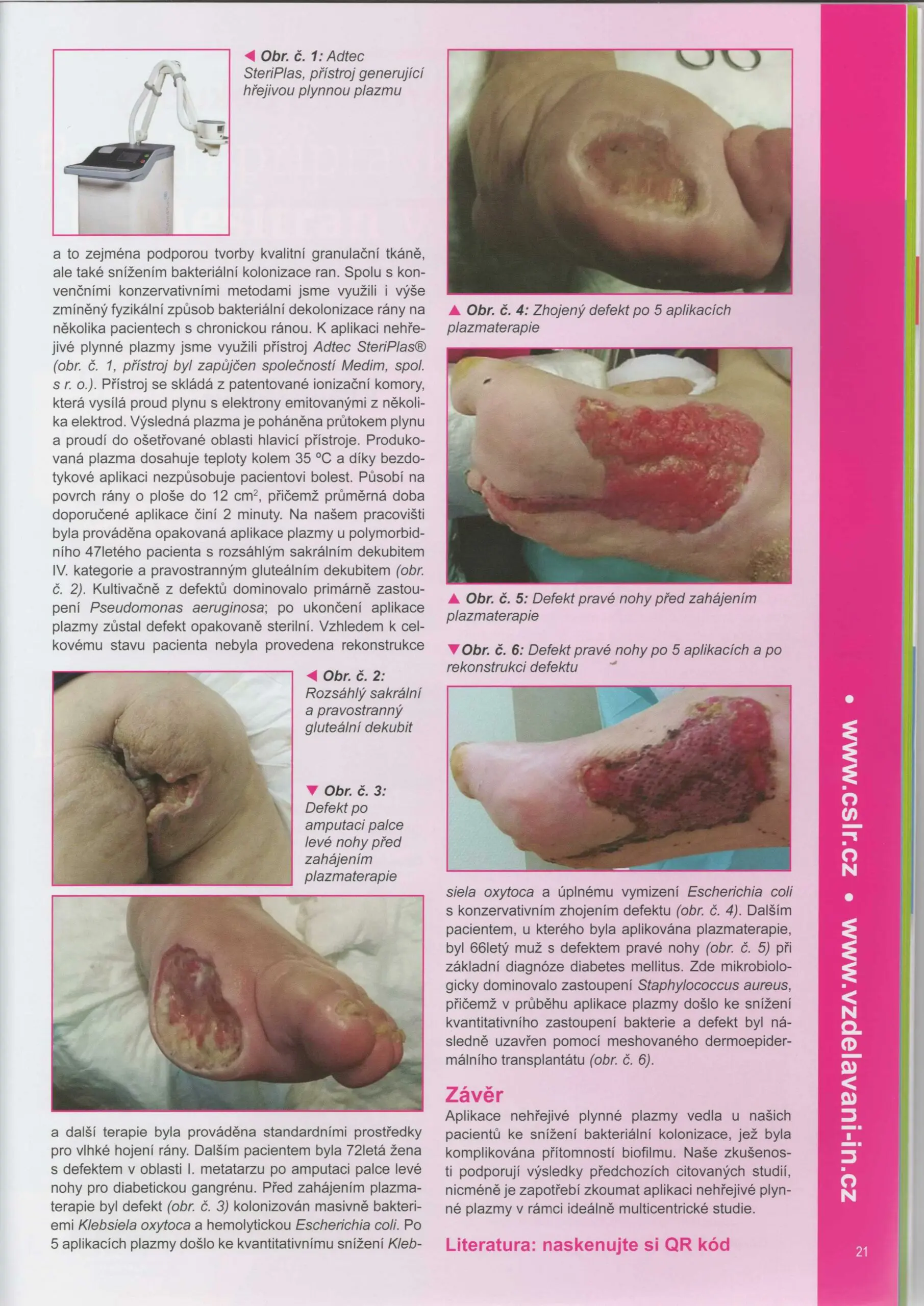
Casopis Lecba ran-page-003
Did you see the Adtec SteriPlas featured in the Czech Society for Wound Healing magazine?
Dr Hokynková from the Department of Burns and Plastic Surgery at Brno Hospital in Czech Republic had published a case study in the magazine to document the efficacy of the Adtec SteriPlas after having tested this on a variety of wounds including pressure ulcers and diabetic foot ulcers.
The article validates the SteriPlas’ ability to significantly reduce microbial load within the wound which allows the body to regain control and accelerate healing of stalled wounds. This includes wounds that are already contaminated with biofilm and listed for amputations. A change from the conventional therapies already existing on the market, the Adtec SteriPlas has proven to reverse further deterioration of complicated wounds and allow full healing and stability to be achieved.
For a translation of the article or to learn more about our efficacy and treatment options, please email us at info@adtecplasma.com


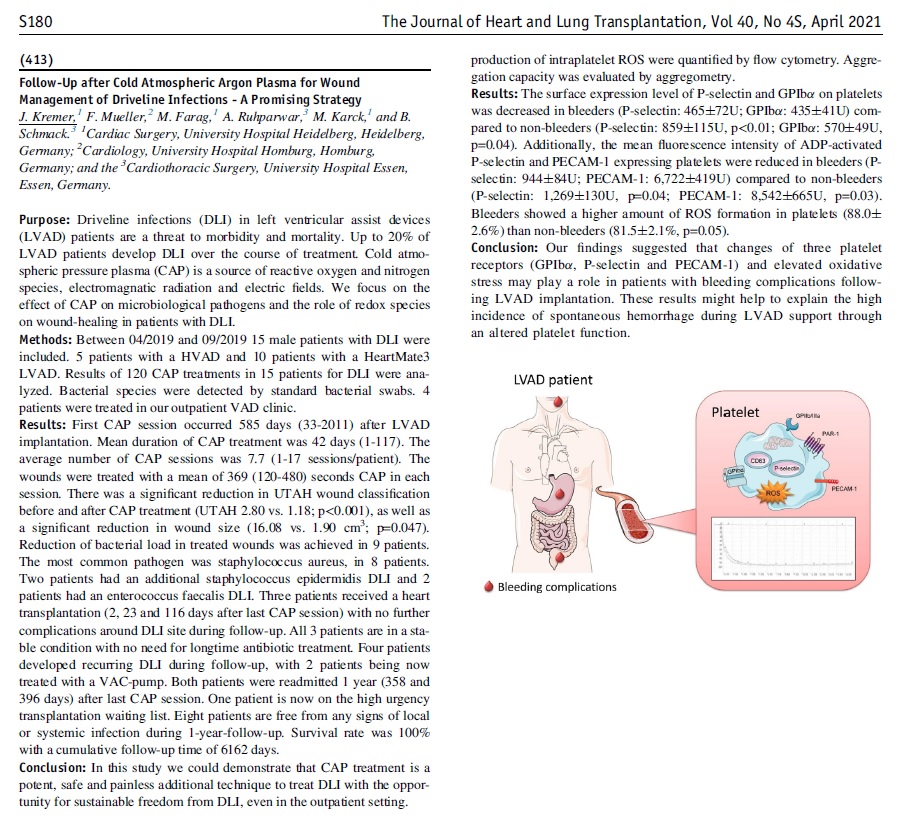
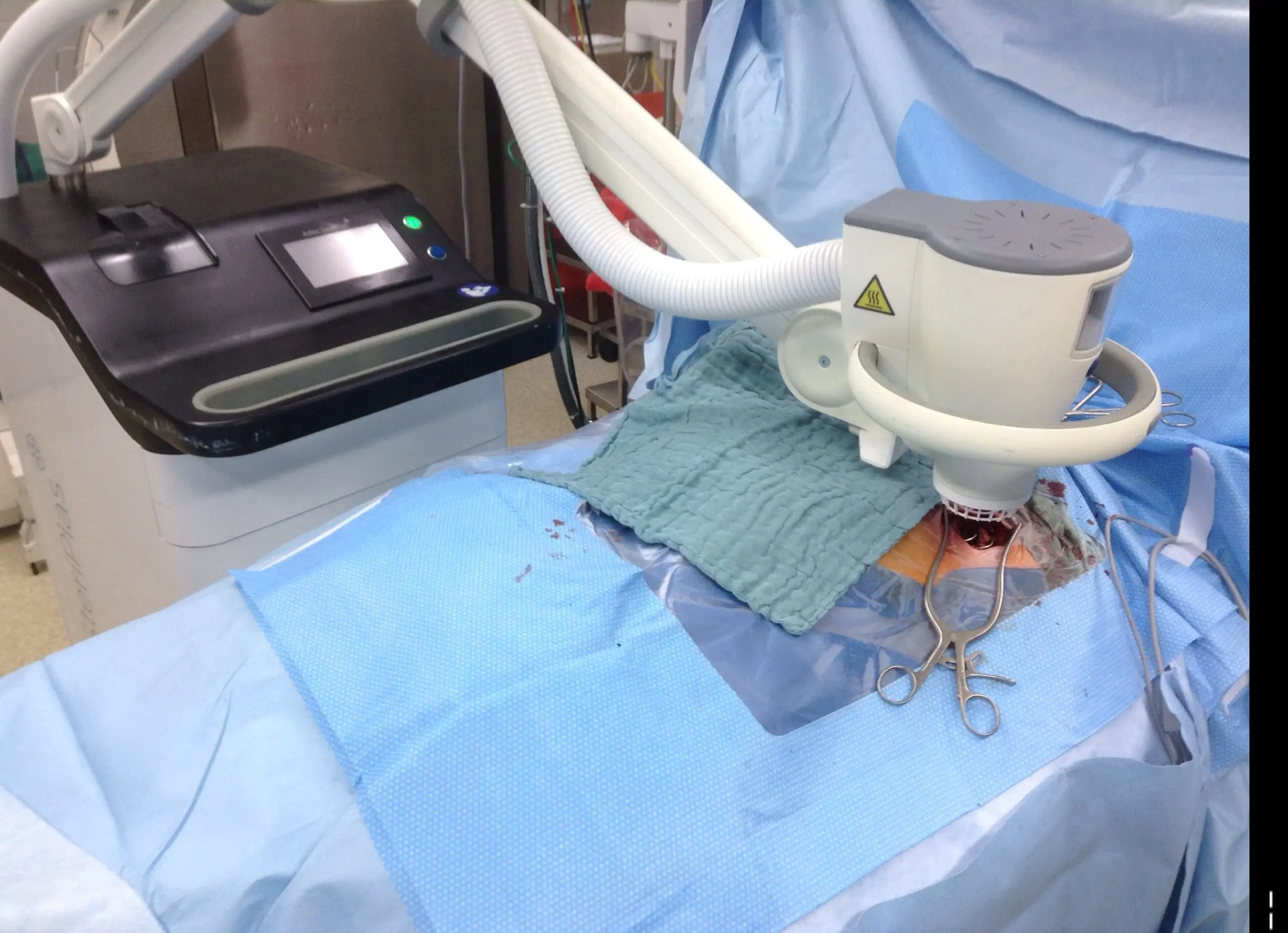
LVAD - Heidelberg publication 2021
Congratulations to Dr Kremer and the Cardiology Team at The University Hospital Heidelberg for their new publication, “Follow-Up after Cold Atmospheric Argon Plasma for Wound Management of Driveline Infections - A Promising Strategy”. This publication features the use of our Adtec SteriPlas medical device for the treatment of driveline infections (DLI). The results demonstrate how the safety, reliability and painless treatment from the Adtec SteriPlas can significantly reduce the wound and infection around the DLI, with the opportunity for sustainable freedom from DLI.
The SteriPlas sets foot in the U.S.A.

We are excited to share news of our collaboration with The Southern Illinois University in United States of America. Our Adtec SteriPlas will be used as part of their study to examine the effect of cold plasma on a variety of infection burn model cases in a research laboratory setting. This study aims to help tackle the outbreaks of antimicrobial resistant microorganisms that continues to be an issue for burns cases. We look forward to providing more information and the results as the study progresses.
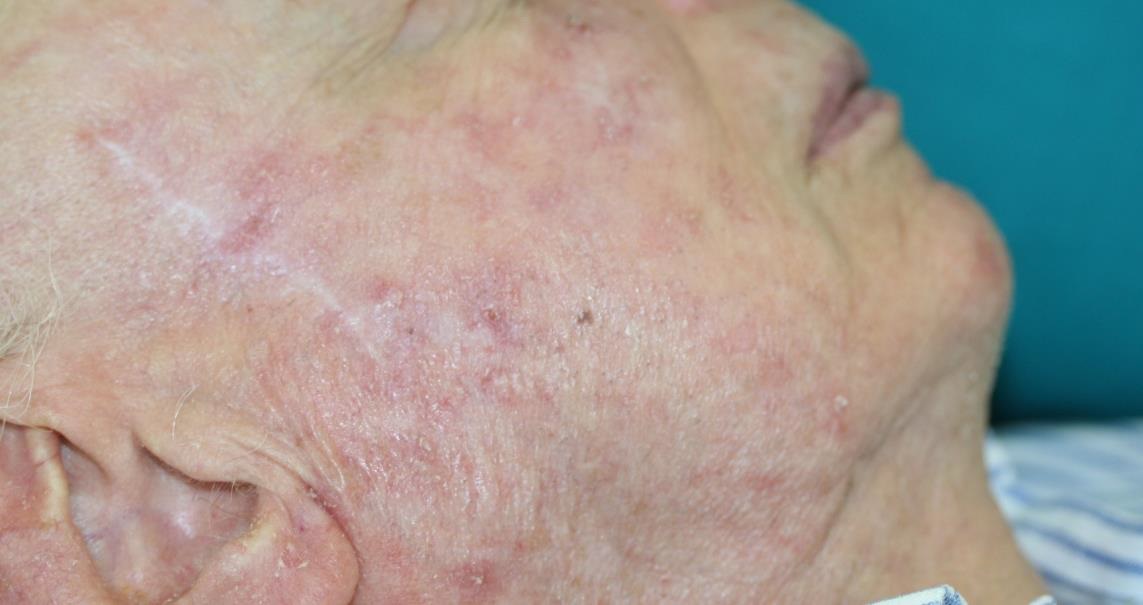
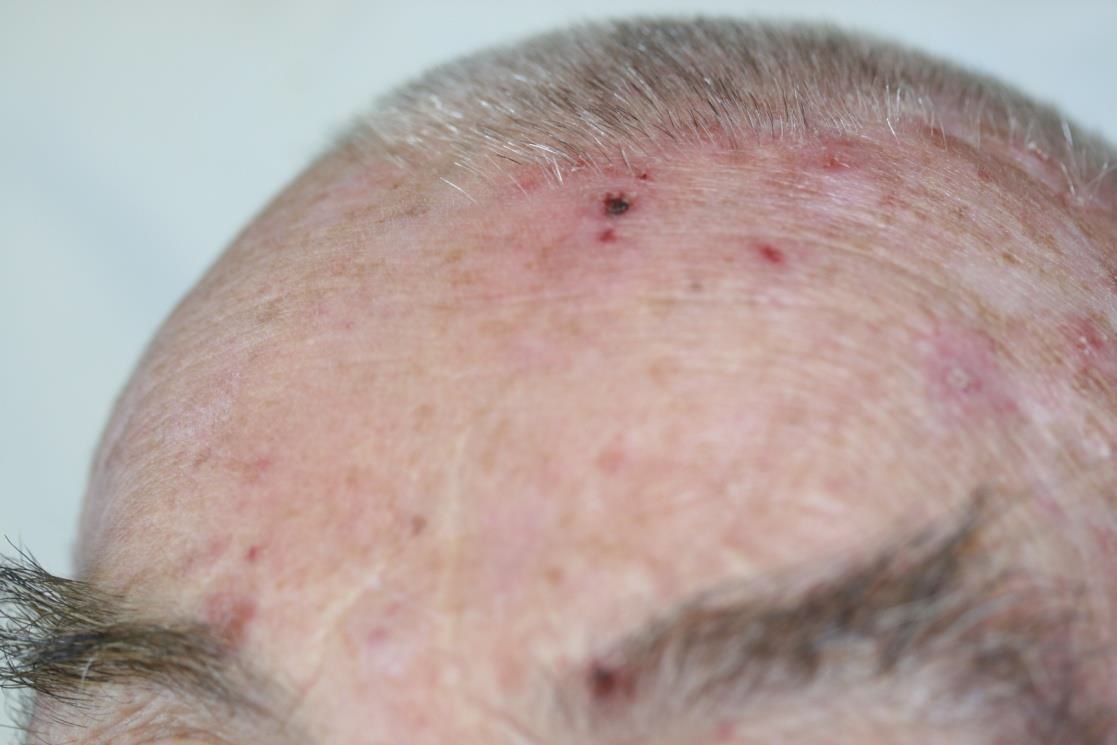
Plastische Chirurgie features the Adtec SteriPlas
Be sure to read our latest advertorial in the Plastische Chirurgie magazine featuring the Adtec SteriPlas for the treatment of actinic keratoses. Our medical device has been thoroughly tested in clinical trials and studies to prove its efficacy for the treatment of complex and impaired wounds, surgical site infections and medical dermatology conditions leading to accelerated healing.
Existing conventional therapies currently on the market for actinic keratoses have sometimes been shown to bear undesirable side effects. So we are proud to boast the safety and reliability of the Adtec SteriPlas with no side effects reported over continuing use since its existence especially for the treatment of actinic keratoses lesions.
To learn more about our medical device, send us an email at info@adtecplasma.com

The SteriPlas can be used to treat a variety of areas on the body
We are proud have been able to treat a wide scale of body areas. Our clinical evidence ranges across a multitude of conditions and areas of the body including the foot, leg, arms, head, neck, scalp, hands, axilla and even groin. All wounds/lesions present in these areas of the body had shown positive responses to treatment with the Adtec SteriPlas.
We are always open to treating new conditions and welcome any study proposals that you have. If you would like to get the Adtec SteriPlas in your clinic or hospital, please contact us at info@adtecplasma.com where one of our team members will assist you with the delivery.
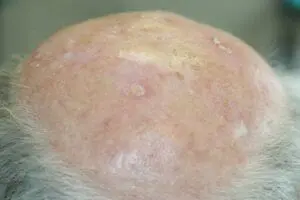
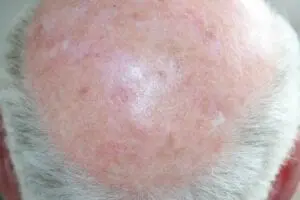
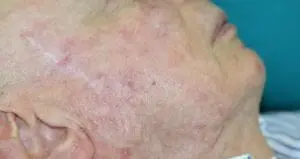
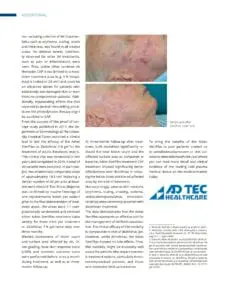
The Adtec SteriPlas featured in another Actinic Keratoses publication
We would like to congratulate Dr Mariachiara Arisi and her team at The Department of Dermatology, Brescia Hospital in Italy for their latest publication: “Cold Atmospheric Plasma (CAP) for the Treatment of Actinic Keratosis and Skin Field Cancerization: Clinical and High-Frequency Ultrasound Evaluation”.
This publication features the Adtec SteriPlas and further demonstrates its efficacy for treating complicated Actinic Keratoses (AK) lesions. All patient enrolled into this study had previously shown resistance or intolerance to conventional field-directed therapies. The results of the study show how all patients who received treatment with the Adtec SteriPlas had significantly reduced AK lesions with the benefit of no side effects observed, quite the opposite for conventional therapies that often bear undesirable side effects. The Adtec SteriPlas continues to be a favoured treatment alternative for actinic keratoses by continuing to be faster-acting, safer and reliable.
You can read the full publication here: https://link.springer.com/article/10.1007%2Fs13555-021-00514-y
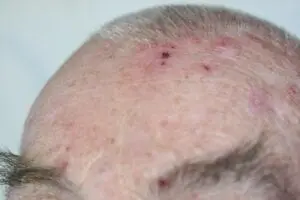
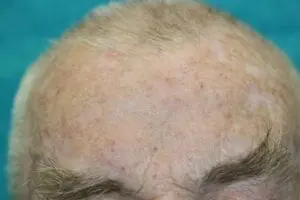
Deep Sternum Surgical Site Infections (DSSIs)
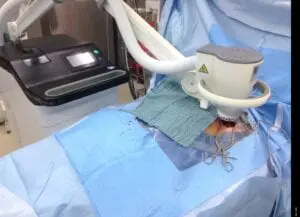
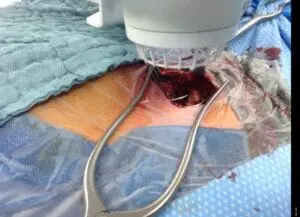
Deep sternal surgical site infections (DSSIs) are a still a severe complication after cardiac surgery. The mortality estimated range is between 15%-40% of all cases. In addition to the significantly extended hospitalization, there is a significant cost burden with a calculated cost of € 36,000 for a single DSSI case.
The use of the Adtec SteriPlas for these complicated conditions has been actively documented as better managing the chronic infections, reducing hospitalization times and cost but more importantly it has shown to decrease the mortality rate. Our medical device has been praised as a tissue and life saving medical device.