Cold Plasma Diabetic Foot Ulcer Clinical Trial Published
Congratulations to Dr Cornelia Wiegand for her published clinical trial paper, “The efficacy of non-thermal gas plasma in the treatment of diabetic foot ulcers stalled by subclinical, biofilm-related wound infection”. This clinical trial paper features the use of our SteriPlas Cold Plasma medical device for the treatment of chronic DFUs infected with biofilm.
The parameters of this study were set much higher than a normal study, with there being a much higher standard of care given (3x per week) vs a typical outpatient setting of 1x weekly.
We wanted to show that even when chronic diabetic foot ulcers are subjected to more frequent outpatient visits with biopsies and swabs included (something not often collected during standard outpatient visits), SteriPlas Cold Plasma still prevails as the successful treatment method due to its physical mode of action and ability to kill all types of bacteria protected within biofilm.
Advanced Wound Care Summit Europe 2022




It was an honour to present at the Advanced Wound Care Summit Europe conference this week. Our extensive knowledge and library of clinical trials and our history as the experienced leader of Cold Plasma medicine was presented to the audience to bring light to the positive benefits of Adtec Cold Plasma devices.
If you would like to hear more about our efficacy and strengths for wounds, surgical site infections and medical dermatology, send us a PM or email us at info@adtecplasma.com
Presenting at The Advanced Wound Care Summit Europe

We’re looking forward to the Advanced Wound Care Summit Europe conference. On the 13th December, our Managing Director, Mary McGovern, will be presenting “Cold Plasma Medicine – Clinical Trials and Regulatory Challenges” where she will explain the importance of clinical trials to test the safety and efficacy of a medical device and the challenges that one can expect.
Since we were the first company worldwide to introduce cold plasma treatment on wounds in clinical trials, it comes to no surprise that we have good experience in this field. Adtec Healthcare boasts a wide collection of clinical trials and studies of our SteriPlas cold plasma medical device to authenticate the efficacy, safety and no side effects claims that we state. We have proven clinical efficacy of managing infections in diabetic foot ulcers and surgical site infections where other treatments have failed.
Evident to our success, this is why Adtec Healthcare’s clinical trials and studies are often referenced by other cold plasma manufacturers who do not have their own clinical studies. With patient safety in mind, clinical trials proving the efficacy must be completed by each manufacturer for the technology used in their device.
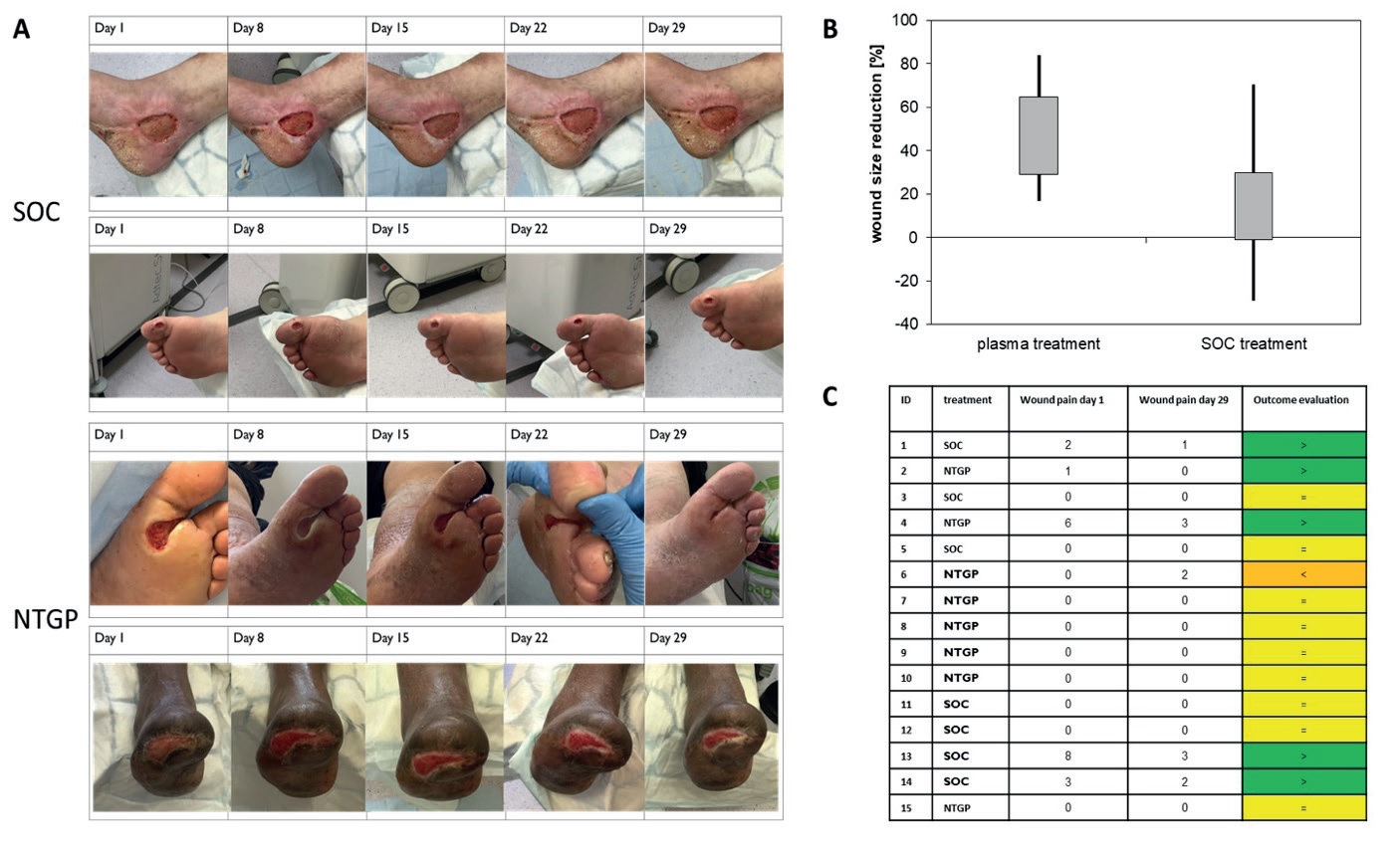
New Wound Management of Driveline Infections with Cold Atmospheric Plasma

We’re ecstatic to see Dr Jamila Kremer’s publication, “New Wound Management of Driveline Infections with Cold Atmospheric Plasma” is now live for public viewing. The paper can be viewed here: https://doi.org/10.3390/jcdd9110405.
The use of our Adtec cold plasma medical device has greatly benefitted her LVAD infection patients treated at the University Heidelberg Hospital since 2019. The results stipulate a stronger infection management advantage over conventional therapy alone for LVAD infections. This is coupled with the benefit of our cold plasma being safe, painless, contact-free and free from side effects.
Driveline infections are the most prevalent infections and reported in up to 60% of LVAD patient cases. Infection of the LVAD system is the fourth most common cause of death within one year after implantation. Dr Kremer’s publication documents Adtec cold plasma treated patients showed a 100% survival rate.
For more information about the benefits of our Cold Plasma medical device, contact us at info@adtecplasma.com
Poster presentation at the DFSG 2022
Did you see Dr Pierides' poster presentation yesterday at the DFSG Diabetic Foot Study Group conference? The poster featured the Adtec SteriPlas success in treating chronic infected diabetic foot ulcers.
The SteriPlas replaced the use of antibiotics for these heavily infected and impacted wounds and had shown remarkable success.

Adtec at the Diabetic Foot Study Group conference in Bratislava
Don't forget to visit us at the Diabetic Foot Study Group (DFSG) conference this weekend. We're having a great time welcoming you to our booth. Our SteriPlas is here ready to give you a live demonstration on how easy it is to use.




TSBIWH Annual Meeting & Symposium in Taiwan
An eventful weekend for our distributor in Taiwan as they demonstrate the strong antibacterial efficacy of the Adtec SteriPlas to health care professionals at the TSBIWH 2022 Annual Meeting and Symposium.






Presentation at the DFSG 2022
We're so excited for exhibiting at the Diabetic Foot Study Group (DFSG) conference next week. Please be sure to visit us at booth no.11 where we will based during the conference. We welcome you to see our SteriPlas Cold Plasma medical device live in action.
We're also excited to announce that the Diabetes Team at Kettering General Hospital NHS Foundation Trust will have a poster presentation illustrating the strong antibacterial efficacy and healing qualities of the Adtec SteriPlas for diabetic foot ulcers.
The poster, "DGH EXPERIENCE OF USING COLD PLASMA MEDICAL DEVICE AS AN ADJUNCT THERAPY TO ANTIMICROBIALS IN TREATING CHRONIC NON HEALING INFECTED DIABETIC FOOT ULCERS" will be presented by Jemma Cruickshank on Saturday 17th September, 13:25 - 14:25.
See you all soon!
The ISHLT discusses the Cold Plasma Project
We’re ecstatic to see our Adtec SteriPlas continue to raise awareness in the LVAD community. A great interview between the ISHLT and Mr Thomas Schlöglhofer from the Medical University of Vienna discussing the benefits of our cold atmospheric plasma medical device for the treatment of LVAD Infections and their broad study being conducted.
Thomas has been an avid user of our medical device for some time now collecting remarkable results with his LVAD patients, documenting the strong benefits of our medical device including the significant reduction of infection rates coupled with full healing, reduction of readmittance and the stabilisation of LVAD systems all with the added benefit of no side effects.
We look forward to the completion of this study and the publishing of the data which will be discussed at the ISHLT 2023 conference next year. We will also be exhibiting at this conference and excited to meet more people from the LVAD community.
https://youtu.be/6-L0sgXLx9o
Antimicrobial (antibiotic) Resistance – the drugs won’t work !
Antimicrobial resistance (AMR) poses a significant global threat of far-reaching proportions. It is estimated that drug resistant infections contribute to nearly 5 million deaths every year and predicted to increase to over 10 million deaths every year. WHO has declared that AMR is one of the top 10 global public health threats facing humanity.
(The worldwide total deaths from COVID is now just over 6 million and we all know how frightening it was before the vaccine was developed)
AMR occurs when bacteria, viruses, fungi and parasites change over time and no longer respond to medicines making infections harder to treat and increasing the risk of disease spread, severe illness and death. As a result, the medicines become ineffective and infections persist in the body, increasing the risk of spread to others. Microorganisms that develop antimicrobial resistance are sometimes referred to as “superbugs”. Without effective antimicrobials, the success of modern medicine in treating infections, including during major surgery and cancer chemotherapy, would be at increased risk. Chronic infections if left untreated could result in tissue damage, amputation, longer stays in hospitals, surgical interventions, or increased possibility of mortality. Patients who are infected with drug-resistant infections are more likely to develop complications and are up to three times more likely to die from the infection. Non-healing wounds in particular, are characterised by complex and mixed bacterial populations, often involving antibiotic-resistant bacteria as well as phenotypically tolerant bacteria in biofilm form. The biofilm factor is clearly of considerable clinical importance: it protects bacteria from antimicrobial agents leading to persistent and difficult to treat chronic infections, and it exacerbates the spread of antibiotic resistance. Surgical Site Infections are also linked to anti-microbial resistance.
SteriPlas cold plasma technology kills bacteria by a physical mode of action and bacteria are therefore unlikely to develop primary or secondary resistance, which we have documented from our clinical studies. SteriPlas cold plasma also kills antibiotic resistant bacteria (e.g. MRSA) and kills bacteria encased in biofilm which are typically up to 1000 times more resistant to antibiotics. SteriPlas has proven clinical efficacy in treating wound infections, diabetic foot infections and surgical site infections in all clinical studies, all with the bonus of no side effects reported.
SteriPlas cold plasma can be used to treat topical infections reserving antibiotics for severe systemic infections.
References
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02724-0/fulltext
https://www.worldometers.info/coronavirus/
https://www.who.int/health-topics/antimicrobial-resistance
https://healthfirsteurope.eu/wp-content/uploads/2020/11/A3A4-48pp-Booklet-Spreads-1.pdf
Bowler, P., Murphy, C. & Wolcott, R. Biofilm exacerbates antibiotic resistance: Is this a current oversight in antimicrobial stewardship?. Antimicrob Resist Infect Control 9, 162 (2020).