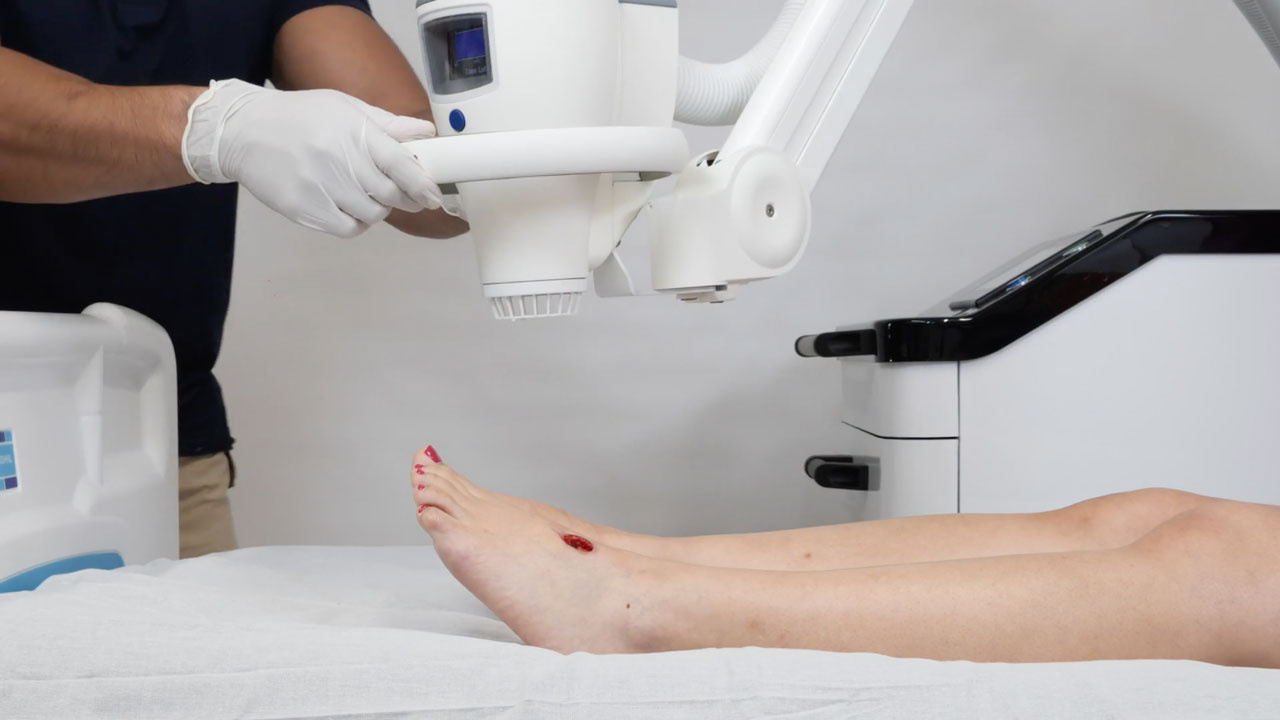
Cold Plasma Wound Treatment
Not all plasmas are
created equal
Often referred to as the 4th state of matter, the
composition of plasma includes heat, OH radicals,
ions, electrons, photons, UV light, and reactive
species of oxygen and nitrogen. While many
assume “plasma is just plasma,” the truth is far
more complex. The creation and delivery methods
of plasma greatly influence its efficacy.
Adtec Healthcare Limited has
been revolutionizing the global
wound care market with the
leading cold plasma technology
for over 10 years. We have
developed the most effective cold
plasma treatment, as proven by
clinical studies. This effectiveness
is a direct result of the specific
blend of plasma that has been
designed and developed.
The different methods
of plasma generation
The unique 'plasma blend' produced by a device is influenced by both the method of plasma generation—whether through radio frequency, microwave, or direct current—and the choice of carrier gas, such as air, argon, helium, or nitrogen. This means that each plasma device is unique.
The specificity of each plasma type means they cater to distinct treatments. It's crucial to understand that equivalence among cold plasma devices is not straightforward, as the plasma characteristics at the treatment site vary. Manufacturers have a professional responsibility to provide clinical evidence unique to their technology, and under EU MDR, product claims should be based solely on this evidence.
Adtec's Premium
Cold Plasma
Our Premium Cold Plasma has been specifically designed to be a denser plasma, making it the most effective plasma in treating wounds.1
All cold plasma will kill bacteria in planktonic free-living state but not all cold plasmas have the required energy and density to kill bacteria in chronic infections.
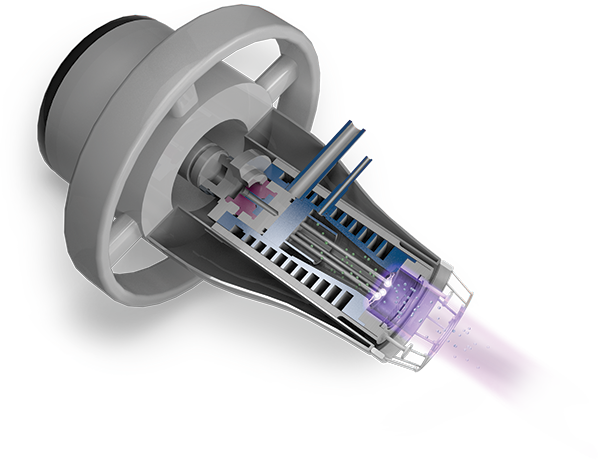
Adtec's patented design harnesses the power of 2.45GHz microwave plasma (high-density) with the reliability of argon as the carrier gas, ensuring safety, control, and consistency.
Why you need
denser plasma
The objective is to deliver a safe dense cold plasma over a wide area with a short treatment time to treat the patient. A denser plasma can penetrate deeper into the wound and heal wounds and infections that other plasmas cannot reach.
We use microwave energy to ignite our argon plasma, which allows us to produce high-density plasmas at reduced voltages. Compared to direct current or DBD, this higher frequency achieves plasma generation with less input power. The lower electric fields associated with high-frequency plasma enhance safety by minimising the risks of electrical leakage and shock.

Greater efficacy:
The only plasma to break the biofilm
While all cold plasmas can target free-living bacteria, not all possess the energy and density for chronic infections as they are unable to penetrate the biofilm. A 5-minute treatment with dense plasma eradicates 100% of biofilm bacteria.3
Given its physical mode of action, bacteria are unlikely to develop resistance to cold plasma making it effective against both biofilm and antibiotic-resistant bacteria, MRSA included.4 Read more
Denser plasma requires a bigger device
For application, the treatment with SteriPlas® head is positioned over the wound, typically lasting between 2 to 5 minutes. Even a sizable wound, measuring 48cm2, can be addressed in just 8 minutes. Unlike smaller delivery systems, the larger head ensures a more effective and uniform treatment, significantly reducing the risk of missing any sections of the wound which can lead to compromised efficacy.
The procedure is non-invasive since plasma does not penetrate skin cells and does not leave any residue or have any unwanted side effects. Furthermore, the treatment is localised, targeting only the bacteria at the infection site leaving other healthy bacteria colonies in the body unaffected. Read more
Leading the way
in cold plasma
Our commitment to innovation and heritage in Semiconductor manufacturing equipment, has culminated in the development of the most potent cold plasma treatment, a claim substantiated by clinical studies. The efficacy of our treatment is a direct result of the meticulously crafted blend of plasma we’ve designed.
“Treating patients with Adtec‘s cold plasma has been one of the most exciting innovations in infection treatments that I have ever had the privilege of being involved in.”
Dr Michael Pierides
Consultant Endocrinologist and Diabetologist
Kettering General Hospital
References
1. Biomed, Perfectus. ‘Anti-Biofilm Activity Demonstrated Following Treatment with a Novel Plasma Device | Perfectus Biomed’, 9 November 2015. https://perfectusbiomed.com/anti-biofilm-activity-demonstrated-following-treatment-with-a-novel-plasma-device-booth-r-cutting-k-f-and-westgate-s-j-2015/.
2. Guo, Julan, Yuxing Huang, Bojun Xu, and Jiao Yang. ‘Efficacy of Cold Atmospheric Plasma Therapy on Chronic Wounds: An Updated Systematic Review and Meta-Analysis of RCTs’. Computational and Mathematica Methods in Medicine 2022 (2022): 5798857. https://doi.org/10.1155/2022/5798857.
3. Potera, Carol. ‘ANTIBIOTIC RESISTANCE: Biofilm Dispersing Agent Rejuvenates Older Antibiotics’. Environmental Health Perspectives 118, no. 7 (July 2010): A288.
4. Zimmermann, J. L., T. Shimizu, H.-U. Schmidt, Y.-F. Li, G. E. Morfill, and G. Isbary. ‘Test for Bacterial Resistance Build-up against Plasma Treatment’. New Journal of Physics 14, no. 7 (July 2012): 073037. https://doi.org/10.1088/1367-2630/14/7/073037.
Distributors
We are interested to partner with a number of key distributors in Europe and globally to ensure that Adtec’s innovative Plasma Products are easy to order and promptly delivered.
Our partnership with distributors reflects our belief that today’s Health Professionals not only rely on quality of product, but dedication to training, education and the infrastructure that ensures a high level of service and support to deliver the highest standards of healthcare.
If you are interested to bring the benefits of Adtec SteriPlas to patients in your region please click the button below.
Clinical
The SteriPlas is a topical antibacterial, cold plasma medical device with proven antibacterial efficacy, proven accelerated healing and a greater advantage over antibiotics for the treatment of wounds, surgical site infections and dermatological conditions. It has been thoroughly tested in an array of clinical trials and publications to demonstrate its clinical efficacy with no side effects reported.
The SteriPlas is a broad spectrum antibacterial (including multi-resistant bacteria) with a unique physical mode of action delivered during treatment. Our studies have shown that antimicrobial resistance is unlikely to be developed.
Our Clinical Trials
Efficacy of cold atmospheric plasma vs. diclofenac 3% gel in patients with actinic keratoses: a prospective, randomized and rater-blinded study (ACTICAP)
F. Koch, K.A. Salva, M. Wirtz, E. Hadaschik, R. Varaljai, D. Schadendorf, A. Roesch (JEADV 2020)
https://doi.org/10.1111/jdv.16735
University Hospital Essen, Germany
Status: Trial completed in 2019 and published in 2020.
Total number of patients in study: 60
Pilot Study on the influence of cold atmospheric plasma on bacterial contamination and healing tendency of Chronic wounds
M. Moelleken, F. Jockenhöfer, J. Dissemond
University Hospital Essen, Germany
Status: Trial completed in 2018 and published in 2020
Total number of patients in study: 37
The efficacy of non-thermal gas plasma in the treatment of diabetic foot ulcers stalled by subclinical, biofilm-related wound infection
C. Wiegand, L. Rudtke, K. Cutting, P. Chadwick, S. Haycocks, D. Russell, N. Dewhirst, J. Woods, S. Jeffery
Salford Royal Foundation NHS Trust, UK
Leeds Teaching Hospitals NHS Trust, UK
Status: Trial completed in 2019 and published in 2023
Total number of patients in this study: 15
Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: Results of an open retrospective randomized controlled study in vivo
Isbary G, et al (2013), http://dx.doi.org/10.1016/j.cpme.2013.06.001
Hospital Munich Schawbing, Germany
Status: Trial completed in 2013 and published
Total number of patients in study: 70
Successful and Safe Use of 2 Min Cold Atmospheric Argon Plasma in Chronic Wounds: Results of A Randomized Controlled Trial
Isbary G, et al (2012), https://doi.org/10.1111/j.1365-2133.2012.10923.x
Hospital Munich Schwabing, Germany
Status: Trial completed in 2012 and published
Total number of patients in study: 24
A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients.
Isbary G, et al (2010), https://doi.org/10.1111/j.1365-2133.2010.09744.x
Hospital Munich Schwabing, Germany
Status: Trial completed in 2010 and published
Total number of patients in study: 36
Randomised placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites
S. Karrer, G. Isbary et al (2013)
University Hospital Regensburg, Germany
Status: Trial completed in 2012 and published
Total number of patients in study: 40
Randomized placebo-controlled clinical trial showed cold atmospheric argon plasma relieved acute pain and accelerated healing in herpes zoster
Isbary G, et al. Clinical Plasma Medicine (2014), http://dx.doi.org/10.1016/j.cpme.2014.07.001
Hospital Munich Schwabing, Germany
Status: Trial completed in 2014 and published
Total number of patients in study: 37
Wounds
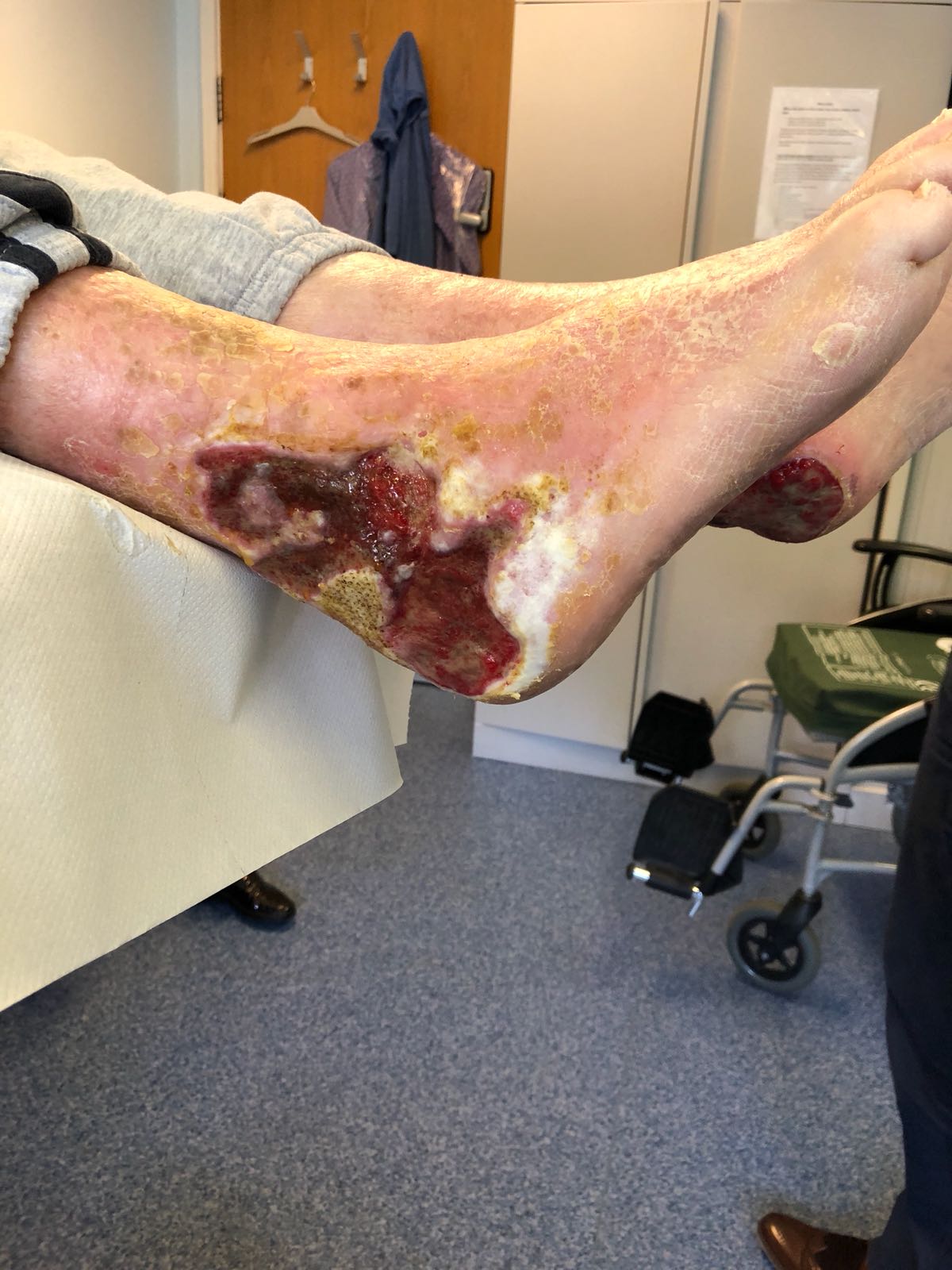
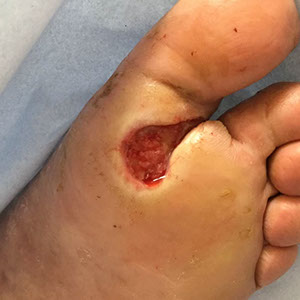
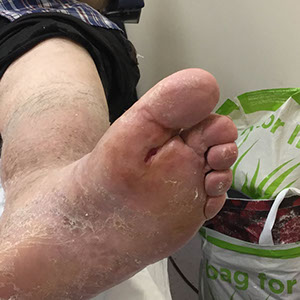
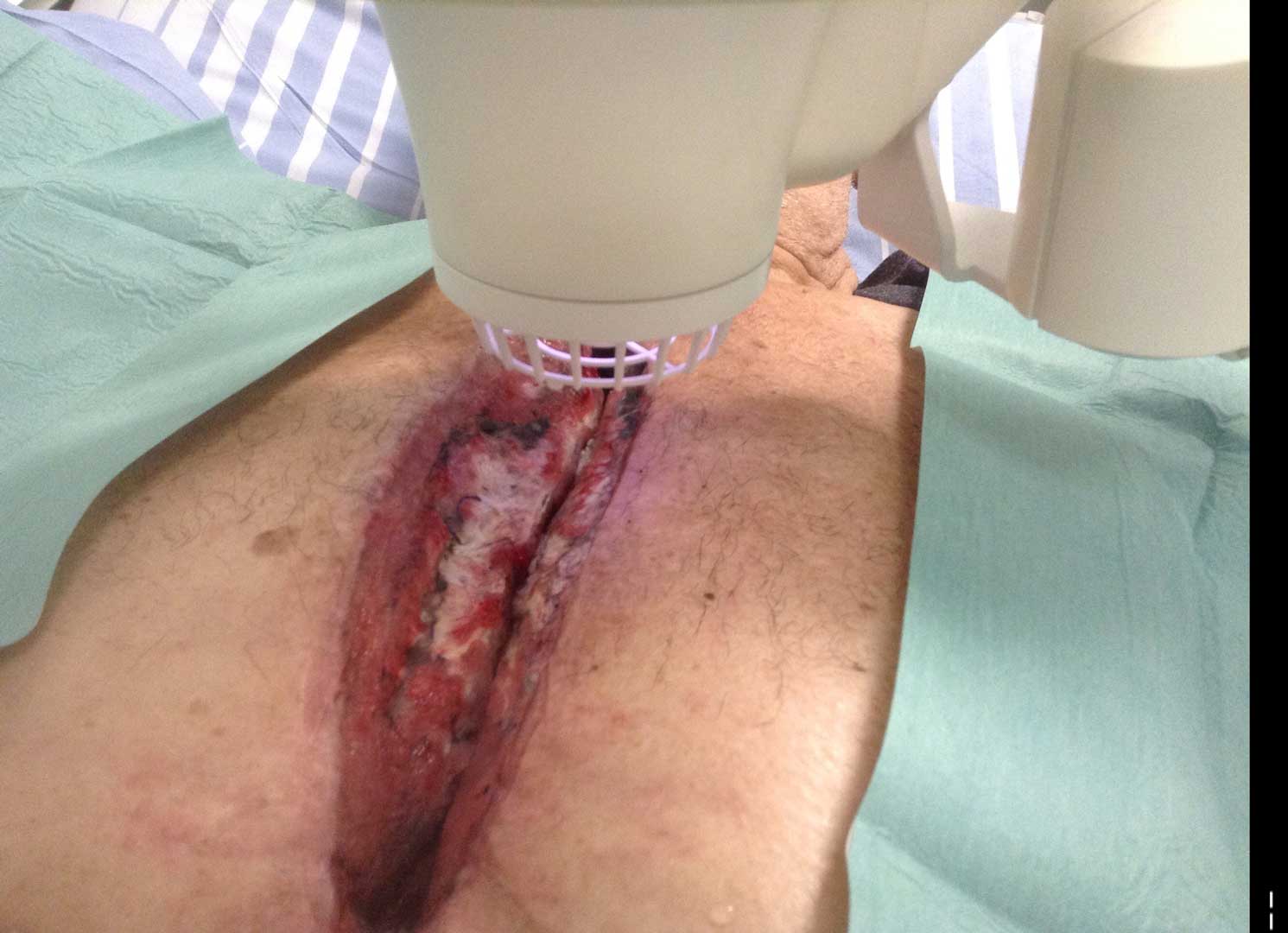
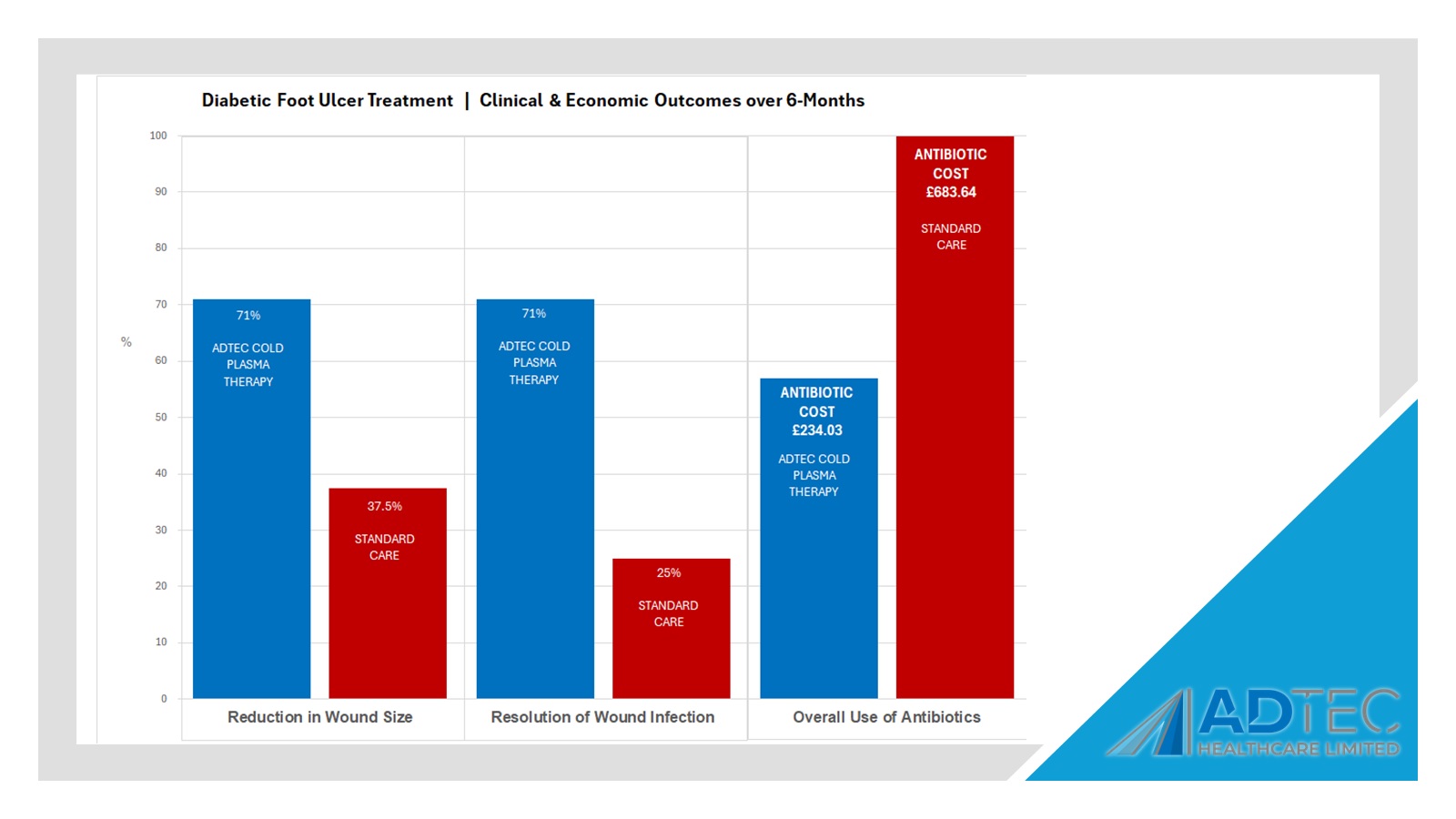
The SteriPlas has clinical efficacy for the treatment of all wound types. Chronic and complex wounds such as diabetic foot ulcers are often stalled by biofilm. Due to angiopathy and neuropathy conditions, poor arterial flow may mean that antibiotic delivery can play very little to no effect to the healing of these wounds. Sadly, the conditions of these wounds can deteriorate leading to the need of an amputation. The cost burden of managing these wounds can also be expensive if using standard treatments alone:
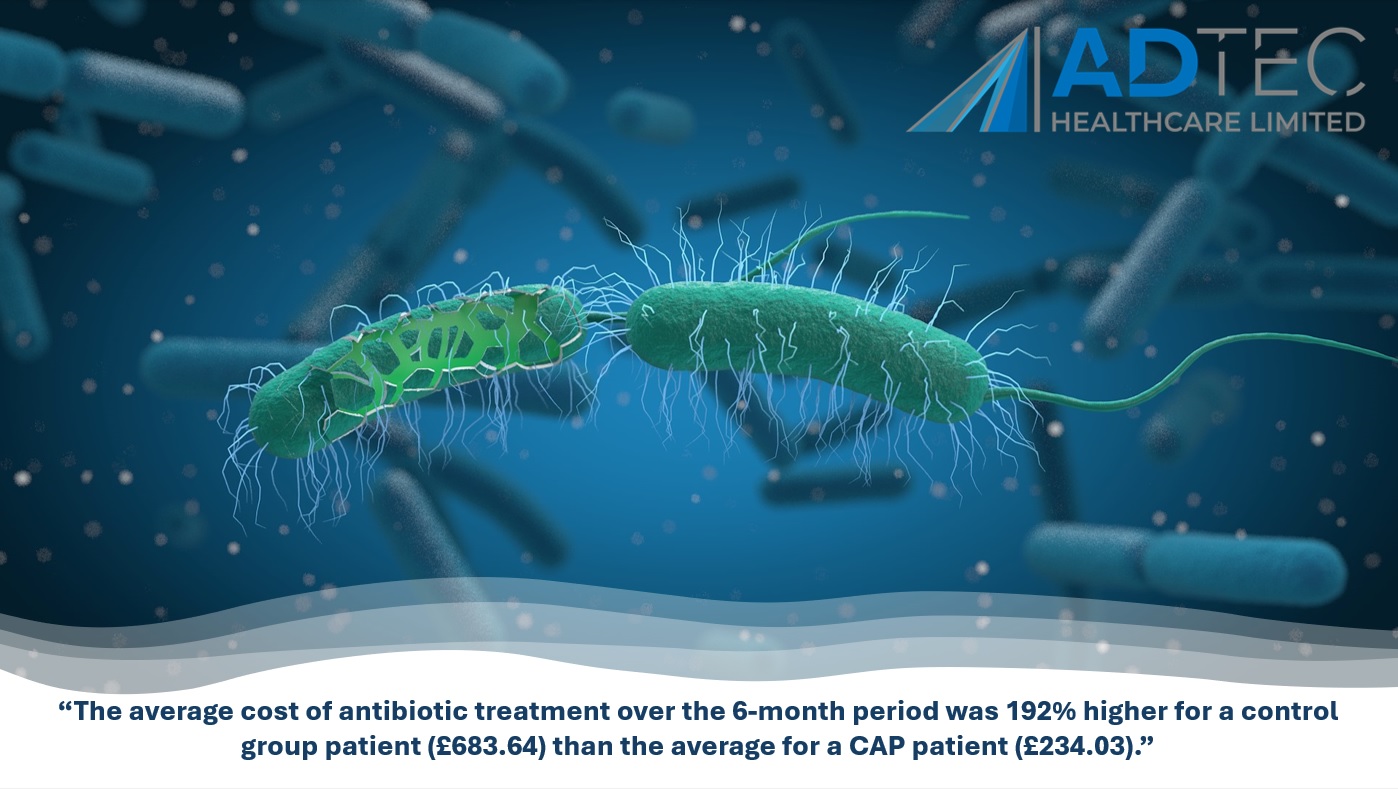
- £7,800 cost per patient for the treatment of diabetic foot ulcer using standard treatment methods over 12 months.
- £16,900 cost per amputation for diabetic foot ulcer patient over 12 months.
The SteriPlas has already been postulated as an alternative to antibiotics. Due to its unique physical mode of action delivered during treatment, bacteria can be easily destroyed without developing resistance to our patented cold plasma. This gives it the treatment advantage over conventional therapies. Treating diabetic foot ulcers with the SteriPlas has shown to accelerate healing as well as be favoured by patients for being contact-free and painless. It has also shown to prevent the need for amputations and save lives. Treatment with the SteriPlas is well tolerated and no side effects have been reported.
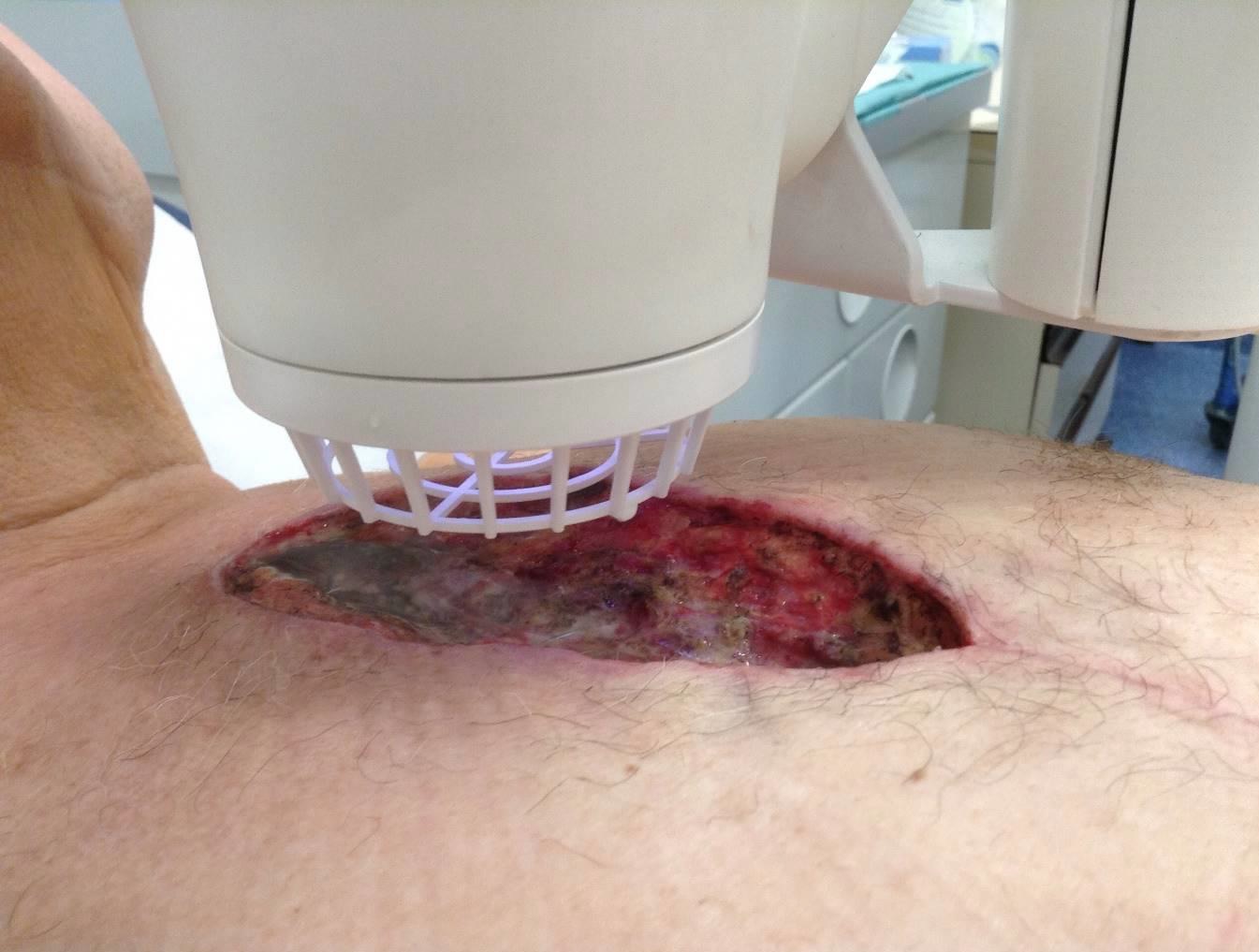
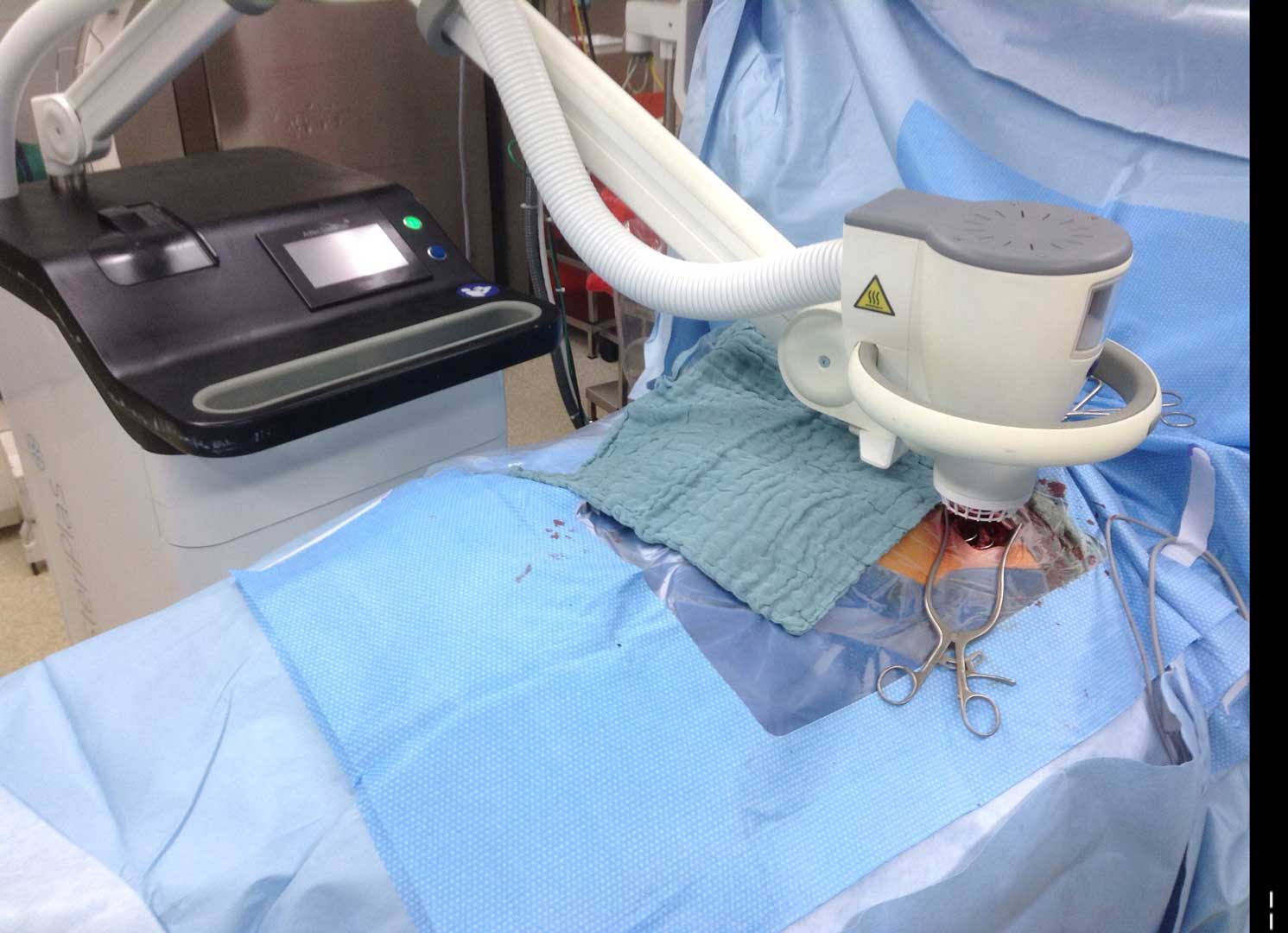
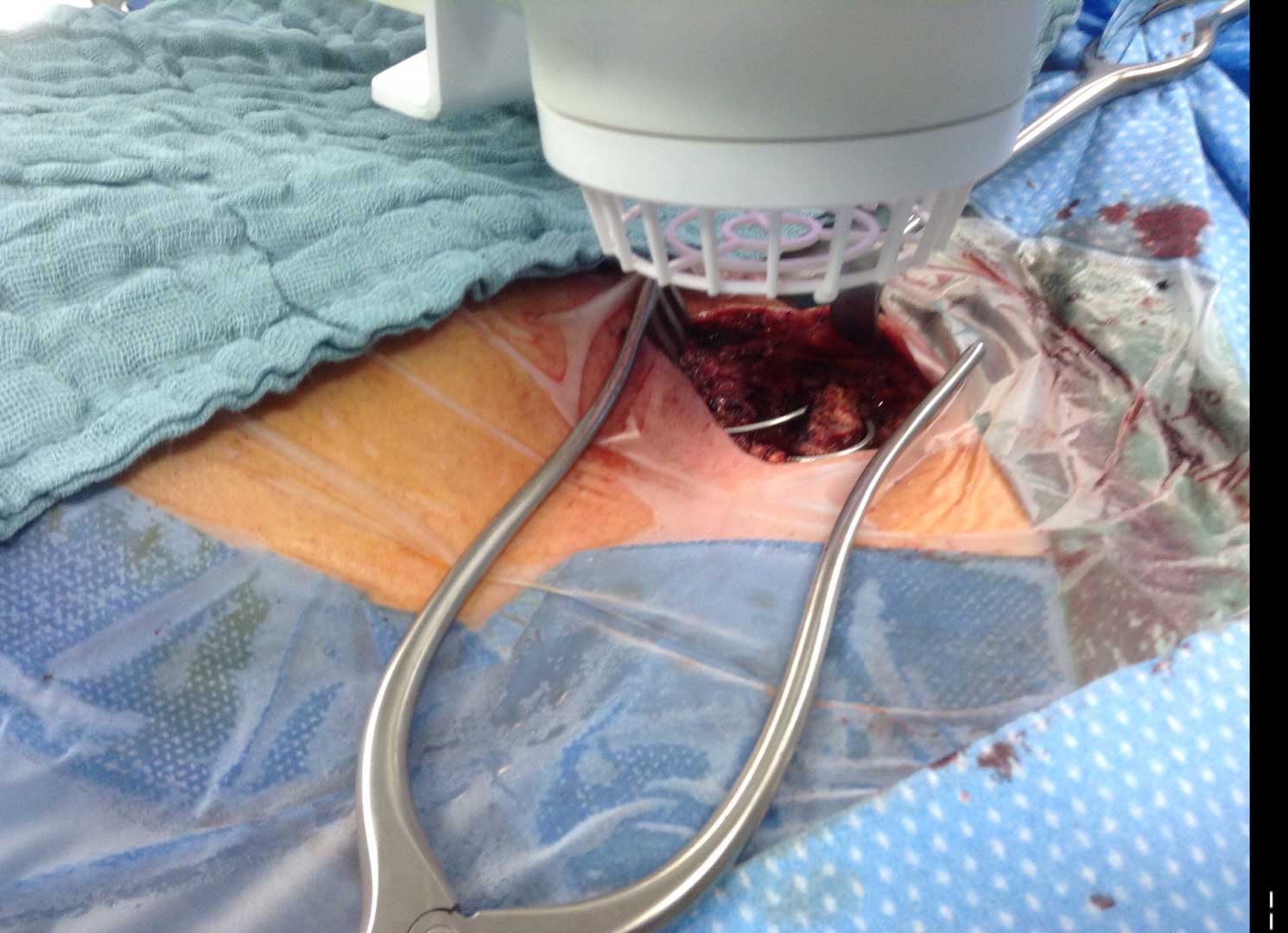
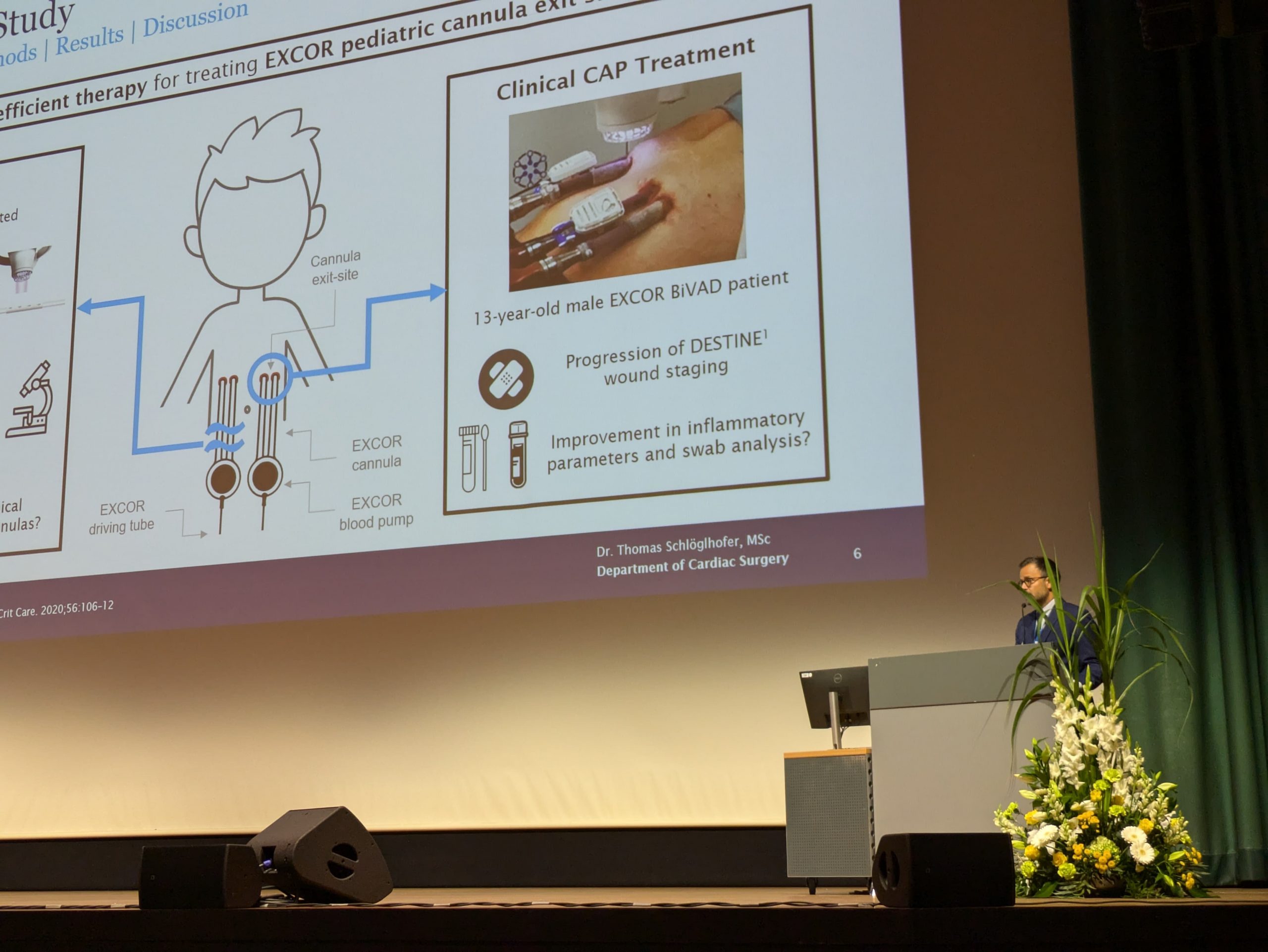
LVAD Driveline Infections

Infected LVAD drivelines may show recalcitrance to treatment with antibiotics and antimicrobial dressings. Antimicrobial dressings are unable to wrap the around infected driveline towards the source of biofilm.
Using the SteriPlas for the treatment of surgical site infections has shown remarkable results in accelerated healing. The gas plasma produced can penetrate through the gaps in crevices around the infected driveline reaching towards the source of biofilm where the bacteria is then destroyed using a physical mode of action.
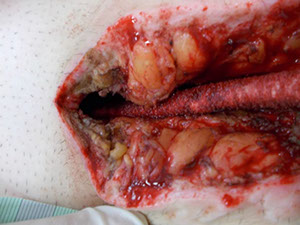
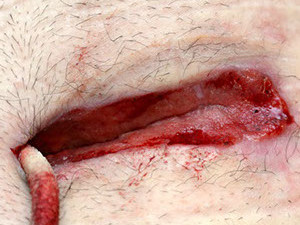
Deep sternum infections are also very complex cardiac wound infections as a result of heart surgery. The mortality rate of patients with sternum infections are relatively high. The SteriPlas has been documented as a tissue and life saving approach to treating these complex wounds, eliminating the need for omentum majus or muscle flap plastic surgery. Treatment with the SteriPlas has shown to accelerate healing in problematic and stalled deep sternum infections or LVAD infections that are complicated with biofilm. The treatment is also well tolerated with no side effects being reported.
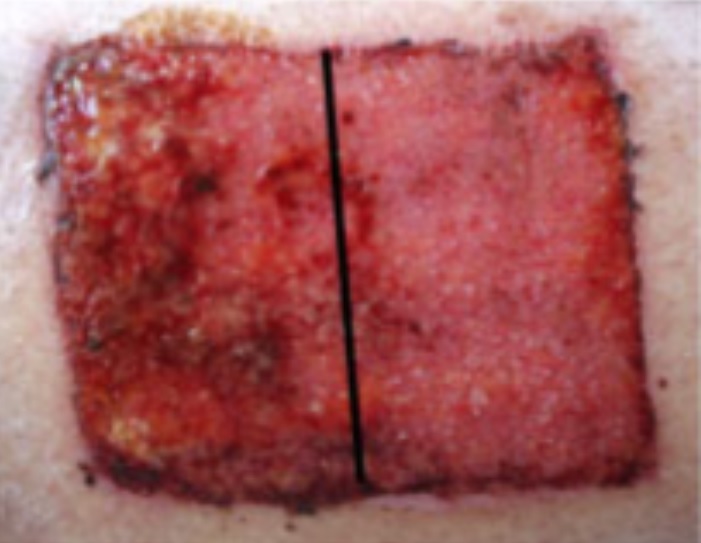
Deep Sternal Wound Infections
Deep Sternal Wound Infections (DSWIs) are infected wounds derived from complications of cardiac surgery. These are typically treated with antibiotic therapy but remain a severe problem in cardiothoracic surgery with typically a high mortality rate.
Due to exposure of the internal chest cavity that significantly increases the infection rate, it is imperative to heal these patients as quickly as possible by least invasive means necessary. As expected, antibiotics are used but in the presence of multi-resistant bacteria, this may play little to no effect.
The SteriPlas has shown to successfully treat these patients by eliminating the infection present.
This offers a new treatment approach and is a tissue saving approach and prevents the need for expensive and complex omentum majus or muscle flap plastic surgery.
Average wound closure time = 16 days of treatment.
Request a Bibliography
FAQs
It has been carefully designed to deliver pain-free treatment including a tolerable temperature of 35°C and contact-free so there is no discomfort touching of the wound like other devices. Due to its gentle treatment most patients favour the therapy.
The plasma generated by the Adtec SteriPlas was also tested for biological safety by research scientists and the results are published. The findings clearly demonstrate for the first time that Argon-Plasma is a safe methodology without inducing mutagenicity in vitro with a treatment time of 5 min and under.
Investigation of toxicity and mutagenicity of cold atmospheric argon plasma Maisch, T. et al
Environmental and Molecular Mutagenesis, Volume 58, Number 3, 1 April 2017, pp. 172-
177(6).
No.
Our patented cold plasma technology has been in use since early 2005 to treat patients from the first Phase I clinical trials to present day. One of our strongest qualities is that there have been no reported side effects or adverse events of our medical device.
The Adtec SteriPlas has been featured in a wide collection of clinical trials and publications. It was the first medical device in history to treat wounds in clinical trials which paved way for further studies from other research enthusiasts. It was also the first medical device in history to test on actinic keratoses lesions in a randomized controlled trial. Our clinical bibliography includes testing and proving the efficacy of the Adtec SteriPlas for the treatment of:
- Arterial ulcers
- Venous ulcers
- Diabetic foot ulcers
- Chronic burns
- Surgical site infections
- Deep sternal infections
- Infected drivelines
- Implantable Electronic Device Infections
- Pump pocket infections
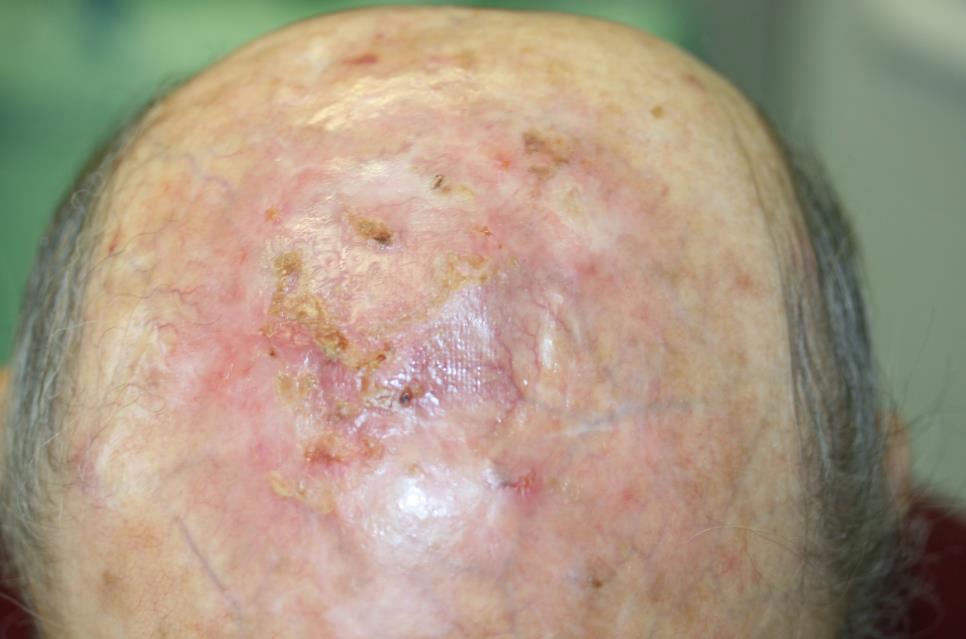
- Actinic Keratoses
- Skin graft donor sites
As the treatment delivered is very gentle, it does not cause any further pain or discomfort that is already experienced prior to the start of treatment. Overtime, the Adtec SteriPlas has shown to increase patient Quality of Life, reduce pain threshold and significantly decrease slough and exudate build up in addition to its main purpose of faster delivering healing rates.
The Adtec SteriPlas is designed as a supplement therapy to standard treatment. Treatments can be conducted in as quick as 2 minutes. This short time is enough to kill the bacteria on the surface of the wound or skin lesion to promote healing.
For deeper infections such as infected drivelines or pump pocket infections, we recommended extending the treatment time up to 5 minutes. This allows the cold plasma to penetrate down the gaps and crevices towards the site of infection.
Debridement is very important for the removal of necrotic tissue and biofilm. It is essential to continue debridement, where necessary, during patient appointments. No supplement therapies (including the Adtec SteriPlas) should replace debridement.
Biofilms can reform rapidly, repeated debridement alone is unlikely to prevent biofilm regrowth; however, effective topical antiseptic application within this time dependent window can suppress biofilm reformation. 1
The Adtec SteriPlas is used in various hospitals across the United Kingdom, European Union, and Middle Eastern countries. Contact us at info@adtecplasma.com to see if it is being used in your local hospital. Alternatively, you can contact us HERE.
We have expressed that accelerated healing in stalled and problematic wounds/skin conditions can be achieved using the Adtec SteriPlas with no reported side effects. Sometimes, we need to demonstrate a proof of concept. If you are interested in testing the Adtec SteriPlas in your hospital clinic, then please contact us at info@Adtecplasma.com. Alternatively, you can contact us HERE.
We have conducted a magnitude of tests and studies sprouting from our initial collaboration with the Max Planck Institute for Extraterrestrial Physics in Germany. From these tests, we have confirmed the safety of using argon gas as the resulting cold plasma generated is reliable and predictable. To achieve a consistent and predictable cold plasma it is ideal to use noble, inert and non-reactive gases such as argon. However, air generated cold plasmas have been shown to be unpredictable and alternate from the surrounding environment (temperature, humidity etc). In return, air generated cold plasmas may create a higher concentration of ozone and reactive species.
Microwave, at 2.45GHz, is considered to be a high enough frequency to stop the ions and electric fields from escaping the plasma chamber. Therefore, microwave generated argon gas plasma will not generate an electrical shock or cause any damage to the target treatment area including the human body.
The Adtec SteriPlas remains having a perfect blend of being safe, reliable, and predictable.
What is Plasma
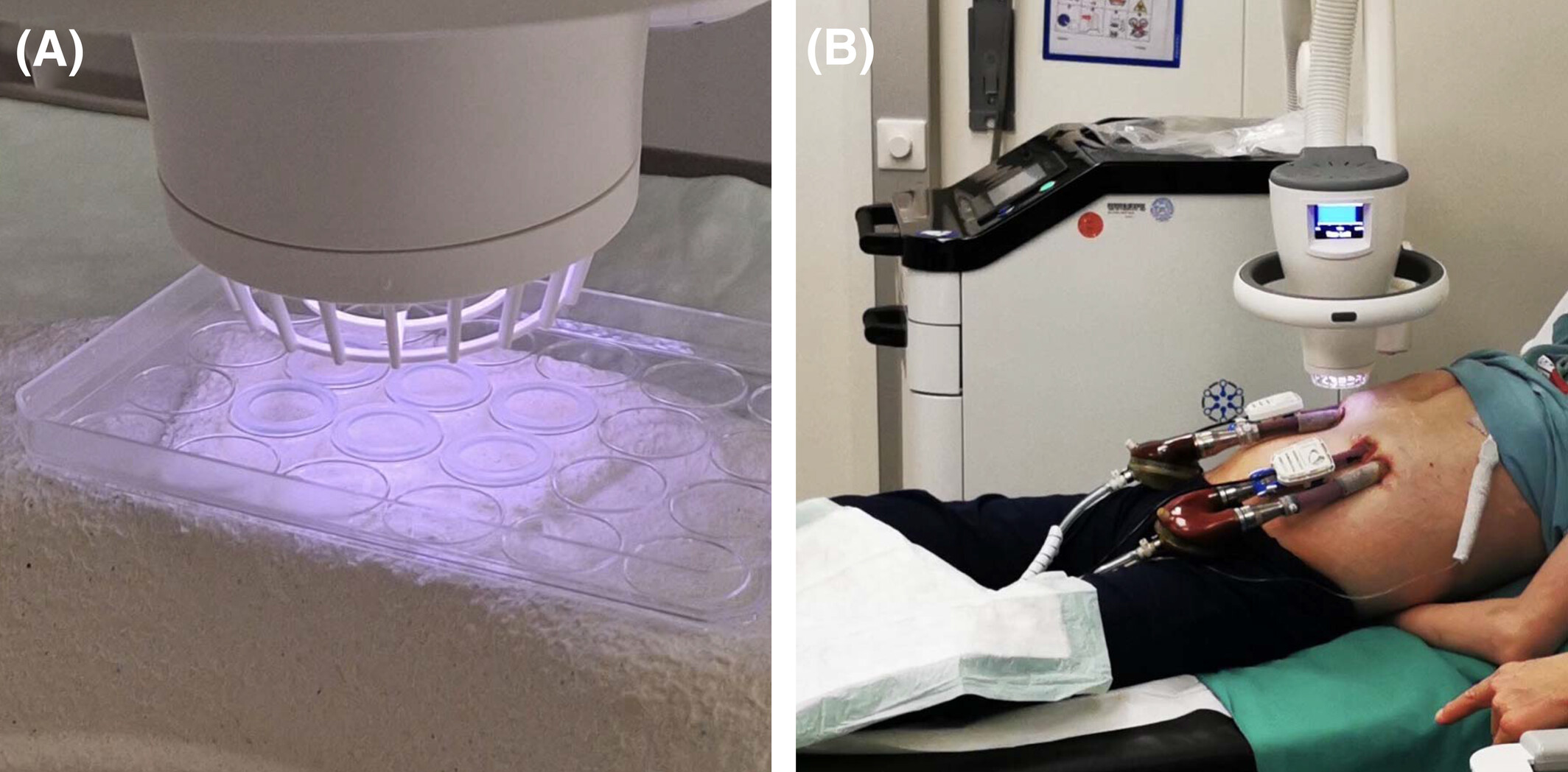
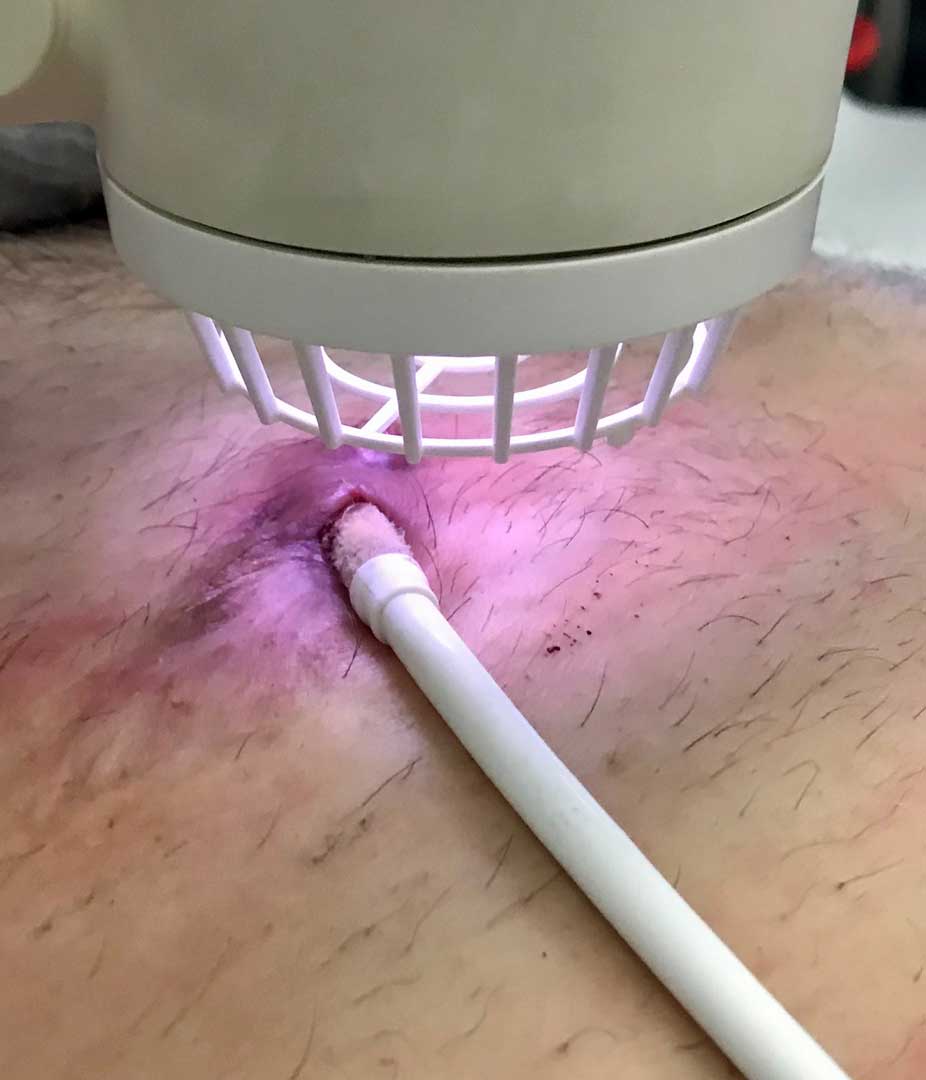
Whilst plasmas are typically known for reaching several hundred to even thousand degrees Celsius, cold plasma such as that observed with our Adtec SteriPlas medical device is well balanced at 35°C. This provides all the antibacterial benefits of plasma at a desirable temperature for patient treatments, offering a safe and effective way to destroy bacteria which would otherwise be resistant to antibiotics.
Cold plasma has a myriad of potential benefits. We have demonstrated through a history of clinical evidence that our patented plasma technology has the benefit to treat patients with infected wounds, surgical site infections and dermatological conditions.
In the photo to the right, our Adtec technician demonstrates the safety of cold plasma by touching the plasma jet.
About us
Our story begins at the birthplace of Adtec: Adtec Plasma Technology Japan (a public company listed under the Tokyo Stock Exchange) with over 30 years’ experience of developing plasma products. From our successes in the semiconductor market for radiofrequency plasma generators and matching units and for also having built a renowned presence as being one of the leaders in this market, we utilized our skills in plasma technology for use in the medical field.
In 2005, Adtec was the first company worldwide to introduce the treatment of cold plasma on chronic wounds for medical use in clinical trials, much of which is still widely discussed today for having paved way for other plasma enthusiasts to broaden their cold plasma studies. Much of what you see today begun from the start of our first clinical trials over a decade ago using our first medical device, the Adtec MicroPlaSter. The results have proved the antibacterial efficacy and accelerated wound healing advantage that our patented plasma technology has over treating patients alone with standard antimicrobial therapies.
The company Adtec Healthcare was later developed and based in London. We are the European subsidiary of Adtec Plasma Technology Japan and we are one of three companies of Adtec’s global presence.
Designed and manufactured in the United Kingdom, our medical devices and plasma products have design and performance improvements that allow safe use in a wider range of clinical applications.
Adtec is committed to providing the highest quality of product and service. From its European Headquarters, Adtec Healthcare’s dedicated sales and technical support staff are available to discuss your next Plasma project, and to help with training, installation or support for Adtec products.
Our Team
Adtec Europe Ltd is a UK company dedicated to the design, application development and manufacture of atmospheric pressure gas plasma products. With plasma expertise and engineering know-how developed in collaboration with our parent company in Japan, we offer customized solutions to our customers. We also provide business development and technical support in Europe for the RF plasma products designed and manufactured by Adtec Plasma Technology Japan.
View our Quality Certificate
Standard Terms of Purchase
Adtec Steriplas
SteriPlas

1. Plasma Treatment Head
Our patented plasma torch where the cold plasma is released towards the treatment site. This features a unique countdown display for remote control of the system from the patient bedside.
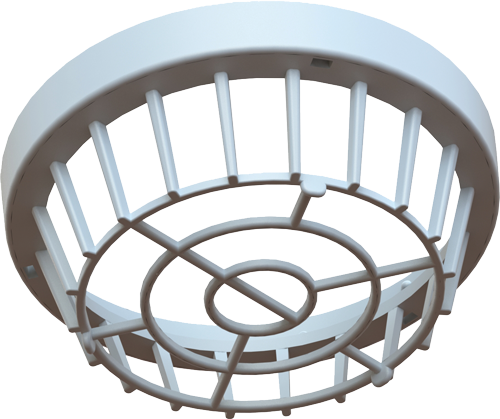
2. Adtec Sensor Module
A single use consumable designed for use between patients. Separating the treatment area from the plasma chamber by 2.5cm allows the creation of reactive species as Cold Plasma is propelled towards the treatment area.
3. Ergonomic handrail
Allows positioning for convenient patient access.

4. Balanced Treatment Arm
Designed for effortless adjustment of the SteriPlas treatment head. Long reach arm extends up to 163cm with ease and smoothness, ensuring position remains fixed over patient treatment area.
5. SteriPlas Control Screen
for treatment regime and configuration.
6. Adtec SteriPlas Console
Manoeuvrable medical device with lockable easy glide wheels, designed for transports between treatment rooms.
What is it for?
In the healthcare industry, there is already an enormous catalogue of topical creams and medical devices used to treat wounds, surgical site infections and dermatological conditions. Some of these therapies are often met with side effects.
With proven anti-bacterial efficacy, the Adtec SteriPlas is used to treat these complex and chronic conditions by accelerating healing whilst giving no side effects to the patient being treated. The Adtec SteriPlas has been shown to restore hope by managing the infection, improving the patient Quality of Life, removing the need to antibiotics and preventing amputations from occurring.
This includes problematic wounds such as diabetic foot ulcers which are considered one of the worst wound types due to the infection with biofilm presence within the wound.
In the field of dermatology, actinic keratoses is a challenging skin condition whereby conventional therapies may often bear undesirable side effects. The Adtec SteriPlas has been proven to accelerate healing of these complicated skin conditions whilst giving no side effects.
How does it work?
Argon gas plasma which includes argon ions, radicals , excited species, electric fields , heat , UV radicals and neutral molecules are propelled out of the plasma chamber with the gas flow by repeated collisions and delivered to the treatment area. As cold plasma is created in the chamber of our the Adtec SteriPlas and are released towards the patient body, the cold plasma also reacts with air during travel to the treatment site and as a result reactive oxygen and nitrogen species are created as by-products. These are all important bactericidal components.
The Adtec SteriPlas delivers a unique physical mode of action during patient treatment. The components of plasma work collectively to destroy the cellular structure of bacteria and microbial DNA. The argon reactive species , heat , UV and ions which have higher energy than argon molecules bombard the bacteria cell wall creating micropores rupturing the cell wall. The cell wall is ruptured by a physical bombardment of the constituent agents, electrical and local heating effects creating micropores. This effectively destroys the bacteria present, even if they are protected within biofilm.
Because of the physical bombardment on the bacteria, a primary or secondary resistance to our cold plasma is unlikely to be developed which we have documented in our wide collection of clinical evidence.
Antibacterial effectiveness
- 73.5% reduction in bacterial load despite the type of bacteria or its resistance to antibiotics
- 50% more effective than antibiotics
- Up to 100% reduction in wound size
- Up to 100% reduction in wound depth
- Up to 100% reduction in perceived level of pain
- Proven to kill a wide range of Gram-negative and Gram-positive bacteria
- No side effects reported
- Quick and simple 2 minute treatment time
- Large 12cm2 plasma treatment area covering a wider infection area.
- Postulated as an alternative to antibiotics
- Reduces the risk of foot and leg amputation
Home
Clinically proven
- 70+ peer reviewed publications featuring the SteriPlas.
- SteriPlas is the only cold plasma medical device with the most clinical trials and strongest efficacy.
Faster Healing Time
- Significantly faster healing rates than conventional therapies such as antibiotics.
- The SteriPlas has shown to heal a chronic wound in as fast as 1 week.
- The only microwave powered, argon cold plasma medical device with the largest 12cm2 treatment area covering a larger wound size than smaller devices.
- Only 2 minutes of the SteriPlas treatment to achieve antibacterial effectiveness.
Combats Antibiotic Resistant Bacteria
- Antibiotics deliver a chemical mode of action where bacteria may develop a resistance towards, e.g. MRSA. Once bacteria develop resistance to antibiotics, treatment options then become very complicated.
- The SteriPlas cold plasma delivers a unique physical mode of action that very quickly destroys bacteria regardless of their resistance profile or if they are protected within biofilm.
- Even in deep and complicated wound infections, the SteriPlas can successfully destroy the bacteria.
No Side Effects
- The SteriPlas is the leading cold plasma medical device with having no side effects reported, with an estimated of 10,000 patients so far treated.
- The SteriPlas comes from a family of Adtec medical devices which was the first ever worldwide to document cold plasma treatment in wounds in clinical trials.
- Since the early 2004, no side effects have been reported as our patented technology remains safe, reliable and consistent as well as being the most effective.
Better Long-term Value
- The only cold plasma medical device with the longest life cycle history.
- Our Health Economics publications have shown significant cost savings can be achieved with the SteriPlas for the treatment of diabetic foot ulcers, sternal wound infections and LVAD infections in comparison to conventional treatments including antibiotics.
- The cost savings achieved can release the cost burden inherited by hospitals and release clinic capacity in overbooked clinics.
Better Patient Experience
- The SteriPlas is safe for the user and patients.
- Treatment is totally contact-free meaning no pressure or pain is experienced as the wound is not touched unlike other devices that require direct application to the site of infection which causes pain and discomfort.
Simple to Use
- A simple plug-and-go approach is all that is required to generate cold plasma with the SteriPlas.
- The cold plasma can be produced within 20 seconds of starting up the SteriPlas.
- The SteriPlas is the only microwave-powered, argon cold plasma medical device that can hover over the wound throughout the duration of the treatment session. Neither the user or patient needs to hold the SteriPlas in position at any point during treatment.

“Infected LVAD drivelines may not respond to treatment with antibiotics and antimicrobial dressings, leading to potentially significantly prolonged hospitalization and additional treatment costs, as well as persistently high mortality rates. Using the Adtec SteriPlas for the treatment of LVAD driveline exit site infections has shown remarkable results in accelerated healing.”
Mr Thomas Schlöglhofer (VAD Engineer)

“My patients are happy when they realize that the treatment is highly effective and it doesn't hurt… it is easy to handle and to move so it is usable in the both operating room and in the intensive care unit.”
Dr Heinrich Rotering (Senior Consultant Cardiologist)

“Wound treatment with cold plasma is certainly one of the greatest innovations in wound therapy in recent years. Here I see a lot of potential in the future for the benefit of my patients".
Professor Joachim Dissemond (Professor of Dermatology, Allergology and Venerology)

“Treating patients with the Adtec SteriPlas has been one of the most exciting interventions / treatments that I have ever had the privilege of being involved in.”
Dr Michael Pierides (Consultant Endocrinologist and Diabetologist)

“In the ACTICAP trial, cold atmospheric plasma (Argon, Adtec SteriPlas®) represented an effective, easily manageable tool for the treatment of actinic keratoses and field cancerization without any side effects…”
Professor Alexander Rösch (Professor of Dermatology)

“We treat patients with hard to heal wounds regularly, some inpatients receive daily treatments, some outpatients are treated as often as they can come with a 2 minute treatment time”
Professor Sigrid Karrer (Senior Physician of Dermatology and Allergology)

"I never thought I would wake up after innumerable years to discover that my foot had healed for the first time"
Patient

“It healed me when antibiotics failed. Now I don’t use antibiotics anymore. No more pills, no more scheduling. This device definitely works”
Patient

“It works when nothing else had!”
Patient
Archives
- March 2025
- February 2025
- January 2025
- December 2024
- November 2024
- September 2024
- August 2024
- July 2024
- May 2024
- April 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
- May 2023
- April 2023
- March 2023
- February 2023
- January 2023
- December 2022
- September 2022
- August 2022
- July 2022
- May 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- December 2020
- November 2020
- October 2020
- September 2020
- June 2020
- May 2020
- February 2020
- January 2020
- October 2019
- July 2019
- June 2019
- May 2019
- April 2019
- February 2019
- January 2019
- September 2018
- July 2018
- March 2018
- October 2017
- August 2017
- July 2017
- June 2017
- April 2017
- March 2017
- February 2017
- August 2016
- June 2016
- March 2016
- September 2015
- August 2015
- July 2015
- June 2015