Presenting at The Advanced Wound Care Summit Europe

We’re looking forward to the Advanced Wound Care Summit Europe conference. On the 13th December, our Managing Director, Mary McGovern, will be presenting “Cold Plasma Medicine – Clinical Trials and Regulatory Challenges” where she will explain the importance of clinical trials to test the safety and efficacy of a medical device and the challenges that one can expect.
Since we were the first company worldwide to introduce cold plasma treatment on wounds in clinical trials, it comes to no surprise that we have good experience in this field. Adtec Healthcare boasts a wide collection of clinical trials and studies of our SteriPlas cold plasma medical device to authenticate the efficacy, safety and no side effects claims that we state. We have proven clinical efficacy of managing infections in diabetic foot ulcers and surgical site infections where other treatments have failed.
Evident to our success, this is why Adtec Healthcare’s clinical trials and studies are often referenced by other cold plasma manufacturers who do not have their own clinical studies. With patient safety in mind, clinical trials proving the efficacy must be completed by each manufacturer for the technology used in their device.
New Wound Management of Driveline Infections with Cold Atmospheric Plasma

We’re ecstatic to see Dr Jamila Kremer’s publication, “New Wound Management of Driveline Infections with Cold Atmospheric Plasma” is now live for public viewing. The paper can be viewed here: https://doi.org/10.3390/jcdd9110405.
The use of our Adtec cold plasma medical device has greatly benefitted her LVAD infection patients treated at the University Heidelberg Hospital since 2019. The results stipulate a stronger infection management advantage over conventional therapy alone for LVAD infections. This is coupled with the benefit of our cold plasma being safe, painless, contact-free and free from side effects.
Driveline infections are the most prevalent infections and reported in up to 60% of LVAD patient cases. Infection of the LVAD system is the fourth most common cause of death within one year after implantation. Dr Kremer’s publication documents Adtec cold plasma treated patients showed a 100% survival rate.
For more information about the benefits of our Cold Plasma medical device, contact us at info@adtecplasma.com
Presentation at the DFSG 2022
We're so excited for exhibiting at the Diabetic Foot Study Group (DFSG) conference next week. Please be sure to visit us at booth no.11 where we will based during the conference. We welcome you to see our SteriPlas Cold Plasma medical device live in action.
We're also excited to announce that the Diabetes Team at Kettering General Hospital NHS Foundation Trust will have a poster presentation illustrating the strong antibacterial efficacy and healing qualities of the Adtec SteriPlas for diabetic foot ulcers.
The poster, "DGH EXPERIENCE OF USING COLD PLASMA MEDICAL DEVICE AS AN ADJUNCT THERAPY TO ANTIMICROBIALS IN TREATING CHRONIC NON HEALING INFECTED DIABETIC FOOT ULCERS" will be presented by Jemma Cruickshank on Saturday 17th September, 13:25 - 14:25.
See you all soon!
The ISHLT discusses the Cold Plasma Project
We’re ecstatic to see our Adtec SteriPlas continue to raise awareness in the LVAD community. A great interview between the ISHLT and Mr Thomas Schlöglhofer from the Medical University of Vienna discussing the benefits of our cold atmospheric plasma medical device for the treatment of LVAD Infections and their broad study being conducted.
Thomas has been an avid user of our medical device for some time now collecting remarkable results with his LVAD patients, documenting the strong benefits of our medical device including the significant reduction of infection rates coupled with full healing, reduction of readmittance and the stabilisation of LVAD systems all with the added benefit of no side effects.
We look forward to the completion of this study and the publishing of the data which will be discussed at the ISHLT 2023 conference next year. We will also be exhibiting at this conference and excited to meet more people from the LVAD community.
https://youtu.be/6-L0sgXLx9o
Antimicrobial (antibiotic) Resistance – the drugs won’t work !
Antimicrobial resistance (AMR) poses a significant global threat of far-reaching proportions. It is estimated that drug resistant infections contribute to nearly 5 million deaths every year and predicted to increase to over 10 million deaths every year. WHO has declared that AMR is one of the top 10 global public health threats facing humanity.
(The worldwide total deaths from COVID is now just over 6 million and we all know how frightening it was before the vaccine was developed)
AMR occurs when bacteria, viruses, fungi and parasites change over time and no longer respond to medicines making infections harder to treat and increasing the risk of disease spread, severe illness and death. As a result, the medicines become ineffective and infections persist in the body, increasing the risk of spread to others. Microorganisms that develop antimicrobial resistance are sometimes referred to as “superbugs”. Without effective antimicrobials, the success of modern medicine in treating infections, including during major surgery and cancer chemotherapy, would be at increased risk. Chronic infections if left untreated could result in tissue damage, amputation, longer stays in hospitals, surgical interventions, or increased possibility of mortality. Patients who are infected with drug-resistant infections are more likely to develop complications and are up to three times more likely to die from the infection. Non-healing wounds in particular, are characterised by complex and mixed bacterial populations, often involving antibiotic-resistant bacteria as well as phenotypically tolerant bacteria in biofilm form. The biofilm factor is clearly of considerable clinical importance: it protects bacteria from antimicrobial agents leading to persistent and difficult to treat chronic infections, and it exacerbates the spread of antibiotic resistance. Surgical Site Infections are also linked to anti-microbial resistance.
SteriPlas cold plasma technology kills bacteria by a physical mode of action and bacteria are therefore unlikely to develop primary or secondary resistance, which we have documented from our clinical studies. SteriPlas cold plasma also kills antibiotic resistant bacteria (e.g. MRSA) and kills bacteria encased in biofilm which are typically up to 1000 times more resistant to antibiotics. SteriPlas has proven clinical efficacy in treating wound infections, diabetic foot infections and surgical site infections in all clinical studies, all with the bonus of no side effects reported.
SteriPlas cold plasma can be used to treat topical infections reserving antibiotics for severe systemic infections.
References
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02724-0/fulltext
https://www.worldometers.info/coronavirus/
https://www.who.int/health-topics/antimicrobial-resistance
https://healthfirsteurope.eu/wp-content/uploads/2020/11/A3A4-48pp-Booklet-Spreads-1.pdf
Bowler, P., Murphy, C. & Wolcott, R. Biofilm exacerbates antibiotic resistance: Is this a current oversight in antimicrobial stewardship?. Antimicrob Resist Infect Control 9, 162 (2020).
EUMS-ISMCS 2022



We are overwhelmed by all the positive interactions we've had at the EUMS-ISMCS 2022 conference. Lots of you have taken a huge interest into our Cold Plasma SteriPlas medical device and its strong efficacy with LVAD infections. It's great to have seen regular faces as well as meeting new.
We hope you also got to see Dr Heinrich Rotering's presentation during the conference, "Treatment of Biofilm-Infested Implants - A Tissue Saving Approach". His great presentation elaborates the key benefits of using the SteriPlas for Sternal wound infections and LVAD infections.
Contact us for more info on info@adtecplasma.com
Welcoming delegates at The Malvern Diabetic Foot conference




We’re having a great time welcoming you all to our booth at the Malvern Diabetic Foot Conference, introducing you all to the benefits of the SteriPlas cold plasma for the treatment of diabetic foot ulcers.
For those wishing to learn more about our clinical efficacy in diabetic foot ulcers, please contact us at info@adtecplasma.com for more information.
Welcoming Cardiothoracic Specialists at The SCTS



We're having a great time at the Society for Cardiothoracic Surgery in GB & Ireland conference, introducing the strong benefits of our Adtec SteriPlas cold plasma for deep sternal wound infections.
Please do pop by & see us at booth 18 to see our medical device in action.
Characterisation of a Cold Atmospheric Pressure Plasma Torch for Medical Applications
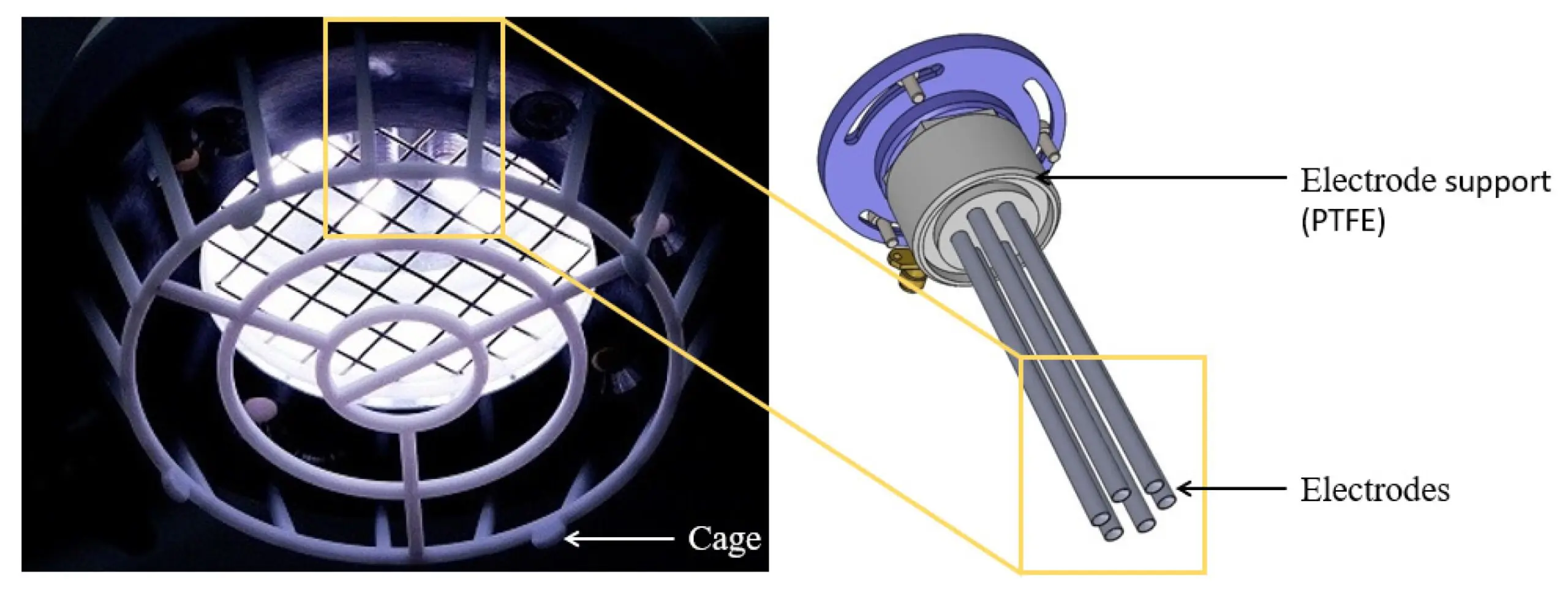
We congratulate Dr Adam Bennett on his most recent publication, “Characterisation of a Cold Atmospheric Pressure Plasma Torch for Medical Applications: Demonstration of Device Safety”.
The safety and effectiveness of plasma devices are of crucial importance, especially for applications where the plasma is discharged near humans. This study presents the novel design and characterisation of a Cold Atmospheric Plasma torch (SteriPlas), which is used in medical applications. The study shows the characterisation methodology that must be undertaken to show that a plasma device is safe, especially when used in an application on human skin. The emission spectrum discharged from the plasma torch is shown. The UV emitted is measured and the effective irradiance is calculated. The effective irradiance enables the determination of the maximum UV exposure limits, which in this application are shown to be over two hours; however, in some applications may be only seconds. NOx and ozone emissions are also recorded. The NOx levels in this application are shown to be orders of magnitude lower than their safety limits and the ozone emissions are also shown to be safe; however, in some plasma technologies the NOx and ozone levels are orders of magnitude higher than the safe levels.
This paper concludes with a discussion of how safety limits vary in different regions around the world and proposes an international standard. It documents the safety of our medical device which further reiterates one of our main strengths where no side effects have been reported.
Access to the full paper can be found here: https://www.mdpi.com/2076-3417/11/24/11864
Adtec Healthcare prepares for exhibition in July

We are very excited to be exhibiting at the Malvern Diabetic Foot Conference between 7-9th July. This will be our first physical exhibition participation in over a year! Be sure to visit us and see our cold plasma medical device live in action, perfect for the treatment of chronic DFUs infected with biofilm.