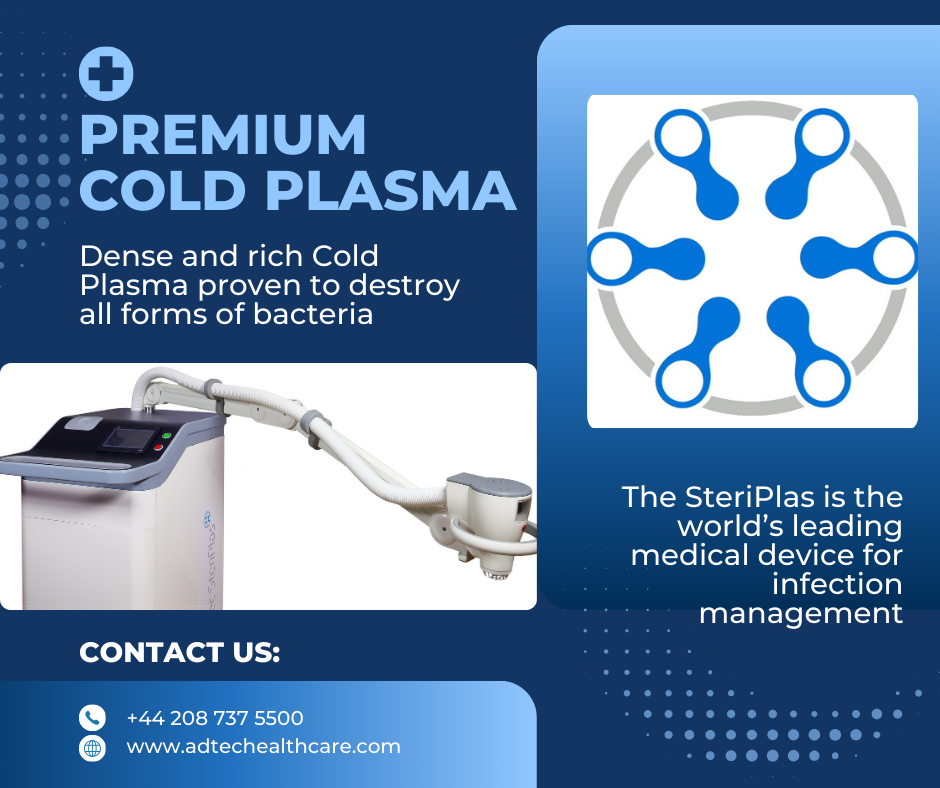
The Science Behind SteriPlas Cold Plasma
What makes the SteriPlas Premium Cold Plasma medical device so powerful in chronic wound care? It's all about cutting-edge technology. We use microwave 2.4GHz technology to generate argon cold plasma, and here’s why this combination is unmatched:
✅ Rich, Dense, and Consistent Cold Plasma: Delivers a premium plasma field for reliable and effective wound care treatments.
✅ Antibiofilm Superiority: Effectively breaks down bacteria protected within biofilms that block healing and resist traditional treatments.
✅ Fast Treatment Time: Just 2 minutes per 12cm² to achieve antibacterial effectiveness—saving time without compromising results.
✅ Safe and Precise: Targeted application with no side effects for patients.
This ideal combination of microwave technology and argon cold plasma ensures superior performance for chronic infection management and wound healing.
We're redefining care with science that heals.
Redefining Global Infection Care
Changing Lives with the SteriPlas Cold Plasma
At the forefront of advanced wound care, our SteriPlas Premium Cold Plasma medical device offers unparalleled benefits for clinicians and patients alike. Here's how we're driving innovation:
✅ Broad Wound Treatment Capability: Effective for diabetic foot ulcers, burns, surgical wounds, autoimmune-related wounds, and more.
✅ Biofilm Eradication: Breaks down stubborn bacteria protected within biofilms that prevent healing—a key advantage in chronic infection care.
✅ Drug-Free Alternative: Reduces or eliminates the need for antibiotics, supporting global antimicrobial stewardship efforts.
✅ Portable and User-Friendly: Designed for ease of use in busy hospital settings or outpatient clinics.
✅ Scientifically Proven: Backed by strong clinical evidence and microwave argon technology to deliver consistently positive patient outcomes.
The SteriPlas is redefining what’s possible in wound management, giving patients renewed hope for recovery.
Together, let’s heal better.
Elevating Wound Care with SteriPlas Cold Plasma

Elevating Wound Care with SteriPlas Cold Plasma
Our SteriPlas Premium Cold Plasma medical device with powerful microwave argon technology continues to transform chronic wound management with innovative features that make a real difference for patients and healthcare professionals alike.
Here’s what sets the SteriPlas apart:
✅ Pain-Free Application: Gentle yet highly effective, the SteriPlas delivers treatment without discomfort for patients.
✅ Non-Invasive Therapy: No need for surgical interventions or harmful chemicals—just clean, advanced plasma science.
✅ Fast Treatment Times: Delivers powerful results with quick, convenient sessions that fit seamlessly into clinical workflows.
✅ Antibacterial Power: Effectively eliminates all forms of bacteria including superbugs.
By offering a safe, effective, and cutting-edge approach to chronic wound care, the SteriPlas is empowering clinicians to achieve better outcomes for their patients.
Revolutionising global Infection Care
Revolutionizing Wound Care with SteriPlas Premium Cold Plasma
When it comes to chronic wound infections, our SteriPlas Premium Cold Plasma medical device is setting the gold standard in advanced care. Here's why healthcare professionals are choosing SteriPlas:
✅ Dense, Premium-Rich Cold Plasma: Our advanced microwave argon technology generates a superior cold plasma that penetrates deeper and delivers a more powerful therapeutic effect than competitor devices.
✅ Biofilm Breakthrough: Effectively tackles biofilm complications, a leading cause of stalled healing in chronic infections.
✅ Versatility: Proven effective across a wide range of wound types—including diabetic foot ulcers, LVAD infections, burns, and post-surgical wounds.
✅ No Side Effects: Gentle yet powerful, patients experience no adverse reactions.
With SteriPlas, healthcare professionals are redefining what's possible for patients with wounds that resist traditional treatments.
Together, we’re transforming the future of chronic wound care.
Why Size Matters

Why Size Matters in Cold Plasma Therapy: How SteriPlas Redefines Safety and Efficacy in Wound Treatment
In the fast-evolving world of medical technology, size often seems to shrink in tandem with innovation. Smaller, more portable devices flood the market, promising convenience and versatility, but sometimes critical aspects of functionality can be compromised. Adtec Healthcare’s SteriPlas, a groundbreaking microwave cold plasma device for wound treatment, boldly defies this trend, emphasizing that when it comes to effective and safe medical treatment, bigger truly is better.
SteriPlas: Leading with Power, Safety, and Precision
At a time when competitors are striving for compactness, the SteriPlas stands out with its larger design, which is purposefully engineered to deliver an unmatched level of therapeutic cold plasma. Unlike smaller Cold Plasma devices, the SteriPlas was built to prioritize treatment potency and safety without compromising on effectiveness. But why exactly does size matter for the SteriPlas? The answer lies in its ability to generate a rich, dense cold microwave plasma field that smaller devices simply can’t match.
Delivering Dense Cold Plasma for Optimal Therapeutic Effect
Cold plasma, a unique, non-thermal form of energized gas, has become a significant breakthrough in wound care due to its ability to kill bacteria. However, the efficacy of cold plasma therapy hinges on both the density and consistency of the plasma field, which is directly impacted by the device’s design.
The SteriPlas, with its larger structure, is engineered to generate and sustain a denser, richer microwave argon plasma field. This dense field ensures that cold plasma thoroughly reaches the treatment area, making it more effective at eradicating harmful pathogens and promoting healing. Where smaller devices might struggle to deliver a consistent plasma , the SteriPlas provides a comprehensive, evenly distributed cold plasma field, amplifying its therapeutic benefits.
Prioritizing Safety with No Side Effects
The SteriPlas was also designed with patient safety as a top priority. Despite its larger size and higher plasma density, it remains exceptionally safe for patients, no reported side effects—a critical advantage in any therapeutic device. Smaller devices may sometimes trade off treatment efficacy to meet compact design requirements, but the SteriPlas’s size is integral to its uncompromising safety standards.
Cold plasma therapy’s expanding use in wound treatment has led to a surge in demand for devices that are not only effective but also safe for prolonged and repeated use. SteriPlas’s larger design enables it to incorporate advanced safety protocols that allow it to maintain a rich microwave plasma field without risking tissue damage or other side effects. This design ensures that the SteriPlas can safely treat sensitive areas and deliver results consistently over time.
Setting a New Standard in Cold Plasma Therapy
The SteriPlas represents a thoughtful balance of innovation, efficacy, and patient-centered design, challenging the notion that smaller is always better. While compact devices may offer some portability, the SteriPlas delivers a level of cold plasma density and therapeutic power that smaller devices cannot replicate. This enhanced efficacy, combined with its rigorous safety measures, positions SteriPlas as an ideal choice for clinics and healthcare providers seeking the best outcomes for their patients.
In wound care, where every treatment counts, the SteriPlas’s larger design is not just a feature—it’s a necessity. It’s a testament to Adtec Healthcare’s commitment to ensuring that patients receive the highest standard of care without compromise.
Conclusion
As cold plasma therapy continues to grow in its applications, healthcare professionals face an important decision in choosing devices that prioritize both treatment potency and patient safety. With its robust, thoughtfully engineered design, the SteriPlas offers an unparalleled therapeutic solution that addresses both needs, setting a new benchmark in cold plasma wound therapy. Adtec Healthcare invites clinicians to explore the SteriPlas difference, where bigger means better, safer, and more effective healing.
Diabetic Foot Ulcer Health Economics

Adtec Healthcare Limited has invested over 10 years in clinical trials and studies to bring you over 80+ peer reviewed studies to validate why the SteriPlas Premium Cold Plasma is the leading medical device in the medical industry for chronic infection management. Not only is our medical device clearly proven to be the strongest against multi-resistant bacteria protected within biofilm but it can treat a wide variety of deep and hard to reach areas that are often recalcitrant to conventional therapies, therefore not limited to superficial wound treatments.
Our recent goal has been to collect the cost effectiveness findings to demonstrate just how the cost burden can be eliminated by the hospital when using our SteriPlas. In the case of chronic diabetic foot ulcers with biofilm presence in the NHS, the SteriPlas has been shown to reduce antibiotic usage by 57% for patients thus relieving the ongoing costs thrown onto the hospital service. Not only are patients healed significantly faster, bed occupancy significantly reduced, and limbs are saved but there is a significant reduction in patient visits to the hospitals thus relieving further costs associated to the hospital.
For more information about our Health Economics data, contact us at info@adtecplasma.com
SteriPlas to be presented at the DFSG conference
Adtec looks forward to supporting Ms Jemma Cruickshank, Antimicrobial Pharmacy Technician from Kettering General Hospital NHS Foundation Trust, at the Diabetic Foot Study Group (DFSG) conference in September.
Jemma has been invited to present the “Retrospective review of the use of argon cold plasma therapy in non-healing diabetic foot ulcers over a 3-year period within a DGH Diabetic Foot MDT Service” at the conference.
Her presentation will include the strong benefits of the SteriPlas on chronic and large diabetic foot ulcers prone to biofilm infections.
The abstract for this presentation can be found here: https://distribute.m-anage.com/from.storage?image=erIsXlaDuNOv64Tv4JyKY6XW3cI1HigkZNgT5cOgYv7Vzt_UHvGbDPesijzVroVO0
For more information about our SteriPlas Premium Cold Plasma medical device, send us an email info@adtecplasma.com
Is all Cold Plasma created equal?

There is no doubt a wide variety of Cold Plasma medical devices currently on the market and new Cold Plasma designs often being released by startup companies. But how do these devices compare in efficacy, strength, reliability, and performance?
To say all Cold Plasmas are the same, which some companies (shockingly) do, would be farfetched and misleading. This is like comparing a race between a sports car and a bicycle or saying all liquids or gases are the same – the product and plasma components are technically very different.
When Adtec first developed Cold Plasma and carried out the first ever clinical trials on wounds many years ago, our data showed the remarkably strong and reputable results that we still deliver today. This is exclusively for our range of Adtec Cold Plasma medical devices. Our data is often cited by other Cold Plasma companies as their own medical devices lack strength in efficacy and performance in clinical data.
The important questions to raise are:
- What is the frequency of Cold Plasma applications per week to achieve full healing?
- How deep can the Cold Plasma penetrate?
- How quick can the Cold Plasma be applied?
- What is the delivery method of Cold Plasma?
- Is Cold Plasma limited to acute and superficial wounds or can it treat deep, chronic wounds with biofilm?
- What is the clinical evidence for the product itself and not cold plasma in general?
Whilst there are some Cold Plasma medical devices that do not satisfy the requirements met by a wound clinician, the SteriPlas does remain the leader and favorable choice in the Cold Plasma medical market. A once per week treatment time of 2 minutes per 12cm2 has been shown to accelerate healing in chronic, problematic, and non-healing wounds with biofilm presence. This cannot be said for all other Cold Plasma devices which recommend treatment once per day and are only indicated for acute, small, and superficial wounds certainly with no biofilm presence.
Due to its overall larger size which houses crucial components ensuring a consistent, reliable and therapeutic Cold Plasma, the SteriPlas is the only microwave-energy, Cold Argon Plasma medical device which delivers deep, dense and rich Cold Plasma able to kill all bacterial species embedded within biofilm. We can boast that no side effects have been reported with our Cold Plasma medical device since the history of its making.
For more information about the SteriPlas and how we have changed the way that wounds are healed, please visit our page: https://adtechealthcare.com/cold-plasma-wound-treatment/
80 Peer-reviewed Clinical Studies

Can the SteriPlas:
Kill all forms of bacteria? YES ✔
Kill bacteria protected within biofilm? YES ✔
Operate safely with no side effects? YES ✔
Treat infected wounds in as quick as 2 minutes? YES ✔
Ready to learn more? Visit our website: https://adtechealthcare.com/cold-plasma-wound-treatment/
Our 80 peer-reviewed clinical trials and publications illustrate why our SteriPlas remains the leader for Cold Plasma with anti-biofilm efficacy
#medicaldistributor #MEDICA2023 #ABHI #Plasma #investorswanted #investor
Day 1 of Medica


Day 1 of the MEDICA Trade Fair was a success. Interacting with many distributors and clinicians with positive interest in our promising Premium Cold Plasma. We encourage you to visit our booth again today to see why the SteriPlas is revolutionising the global wound care market.
Don't forget to attend our presentation today from 11:55 - 12:15 at the ABHI stand. We will be presenting the strong clinical evidence of the SteriPlas Premium Cold Plasma used for wounds, Surgical Site Infections and medical dermatology in the UK Theatre in Hall 16, J48.