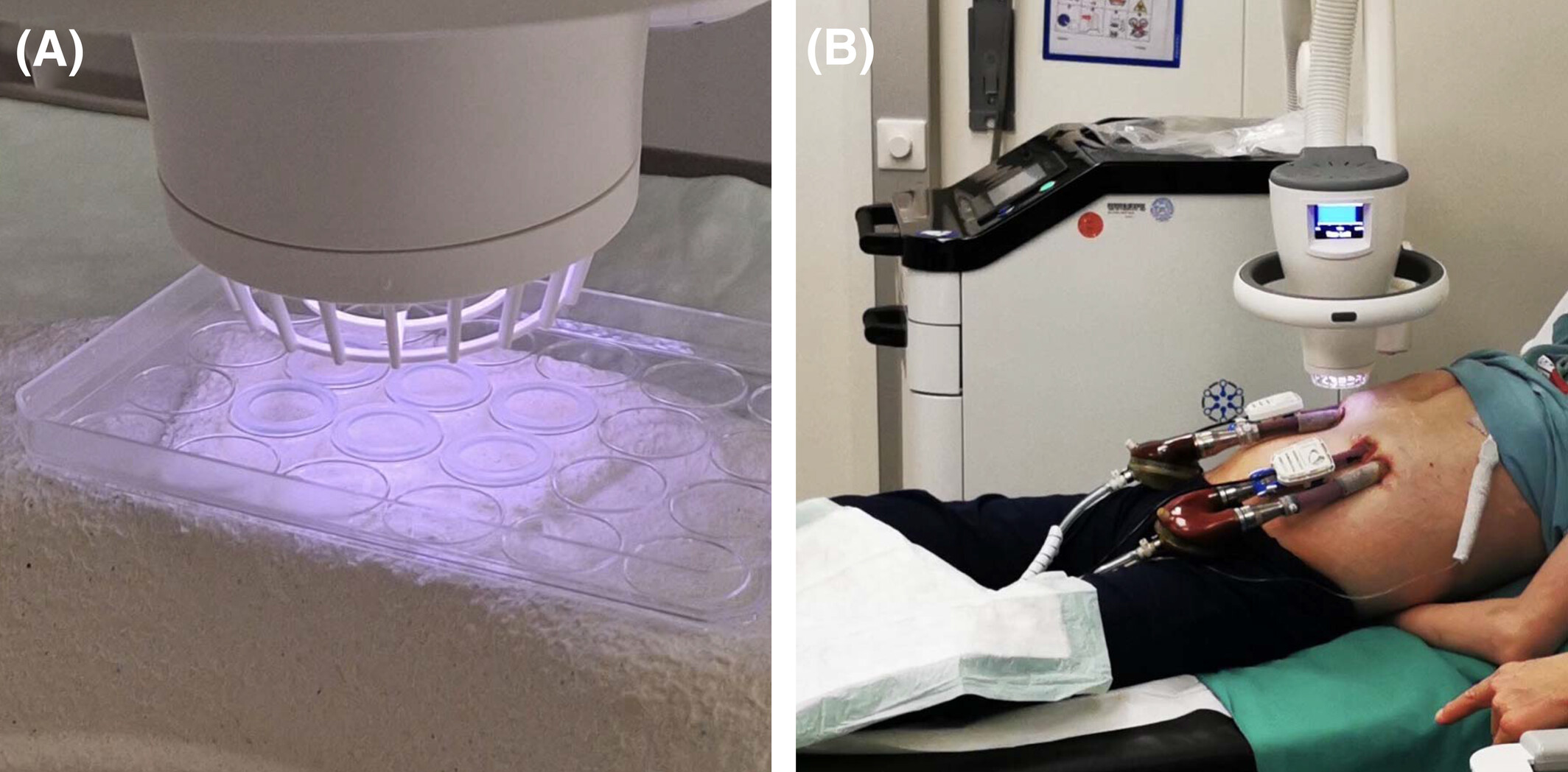
First ever recorded study for CAP on Pediatric Cannula infections
🚨 Exciting News! 🚨
We are thrilled to share a groundbreaking study by Dr. Schlöglhofer, recently published in the Artificial Organs journal from Wiley. This study highlights the remarkable safety and efficacy of using SteriPlas Premium Cold Plasma for managing infections on Berlin Heart EXCOR cannulas.
🌟 This is the first publication to demonstrate the potential of CAP therapy for treating infections and inflammation in pediatric patients with paracorporeal pulsatile VADs.
Key findings show that SteriPlas treatments:
- Do not alter the surface structure of cannulas, as confirmed by SEM micrographs.
- Lead to significant improvements in DESTINE wound staging.
- Drastically reduce bacterial load and inflammatory markers.
- Achieve these results with no observed side effects.
This study marks an exciting step forward in infection management for pediatric VAD patients. To read the full publication, visit: Wiley Library.
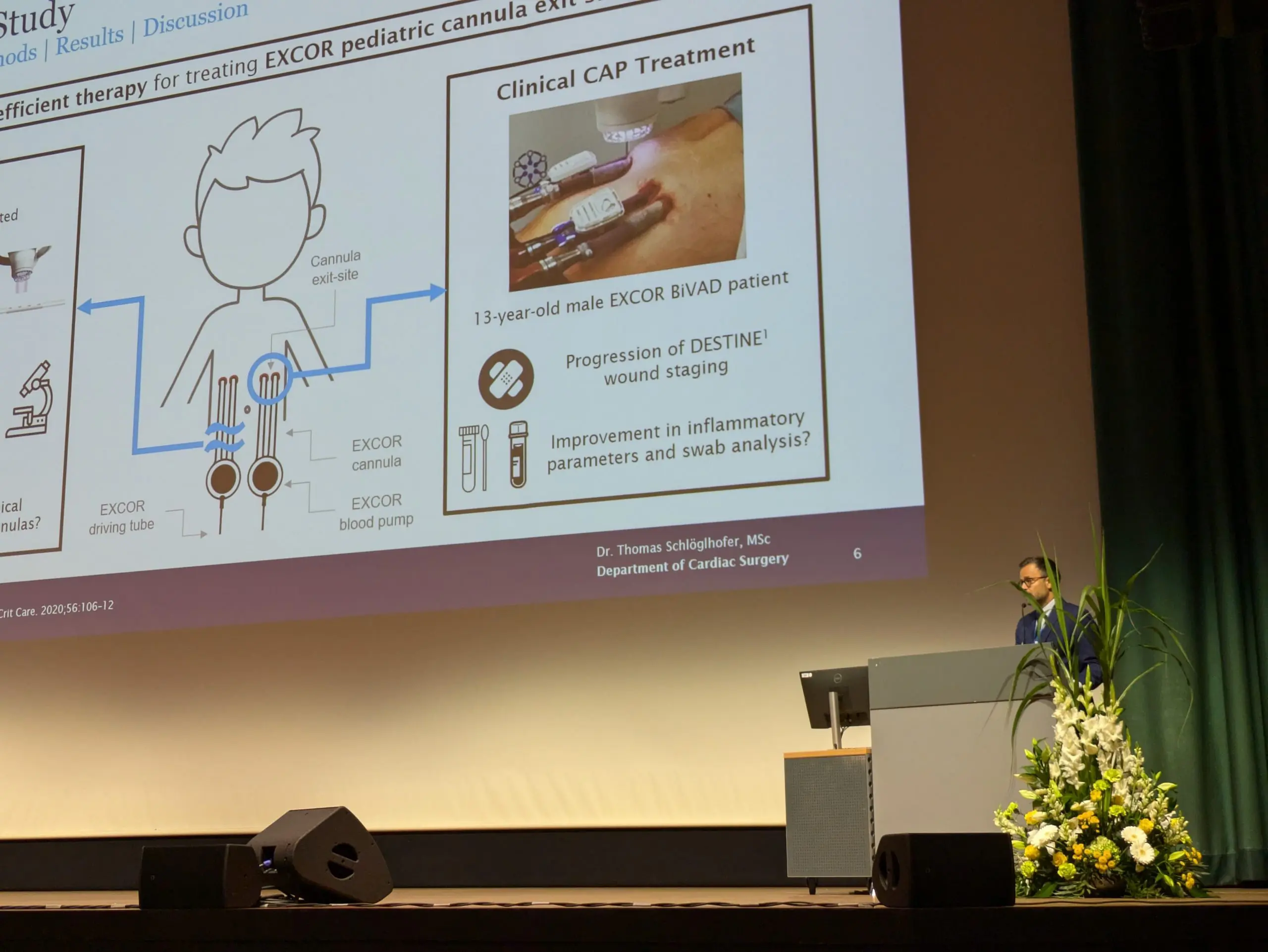
Dr Schlöglhofer presents the SteriPlas at ESAO 2024


A huge congratulations to Dr Thomas Schlöglhofer for his presentation at the European Society for Artificial Organs 2024 conference.
His presentation, “Cold Atmospheric Plasma Therapy as a Novel Treatment for Berlin Heart EXCOR Pediatric Cannula Infections” features the use of our SteriPlas Premium Cold Plasma for infection management on Berlin Heart EXCOR cannulas in Pediatric patients.
We thank Dr Schlöglhofer for his work towards this study and for recommending the SteriPlas as the only Cold Plasma device for the infection management of Berlin Heart EXCOR products.
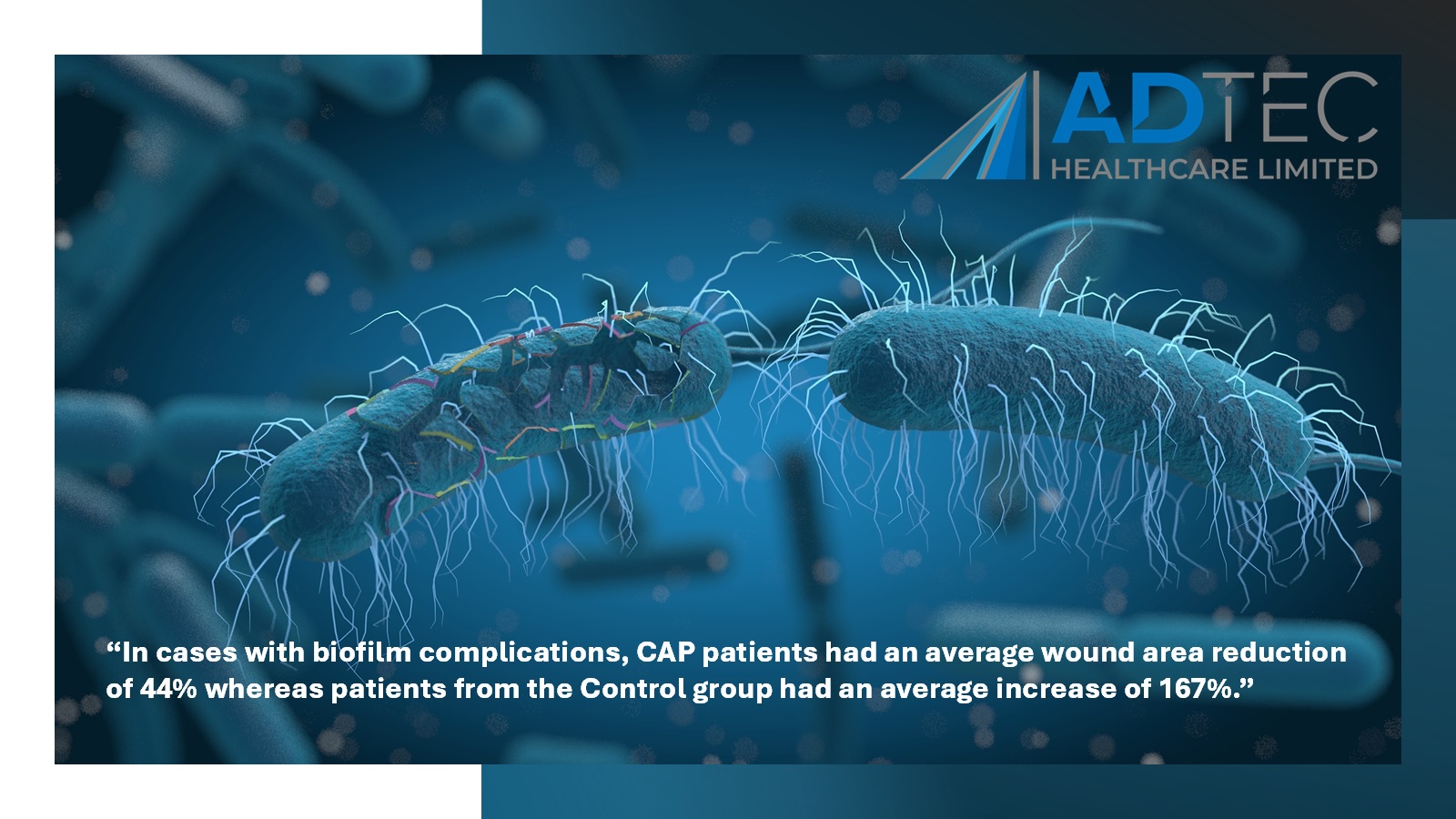
SteriPlas proven to heal patients faster
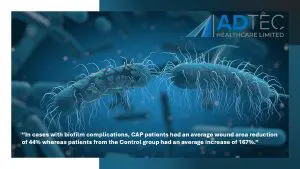
“In cases with biofilm complications, CAP patients had an average wound area reduction of 44% whereas patients from the Control group had an average increase of 167%.”
Diabetic foot ulcers complicated with biofilm are already prone to poor arterial flow, therefore, the delivery of antibiotics to a complicated site already infected with multi-resistant bacteria may pose little to no help in healing the wound. This unfortunately can lead to amputations and further complications.
The SteriPlas benefits significantly to antibiotics due to its unfailing physical mode of action delivery during treatment. Multi-resistant bacteria, whether embedded and protected in biofilm, is quickly destroyed in as quick as 2 minutes. The treatment is delivered directly at the site of infection, is painless, contact-free, and most importantly has no side effects. As bacteria is destroyed so easily with our Premium Cold Plasma therapy, patients are healed and relieve the costs burden associated to hospitals.
“The SteriPlas is a cost and clinically effective treatment option for diabetic patients with infected chronic lower leg wounds in the absence of significant peripheral arterial disease.”
For more information about our strong clinical efficacy and #HealthEconomics, contact us at info@adtecplasma.com
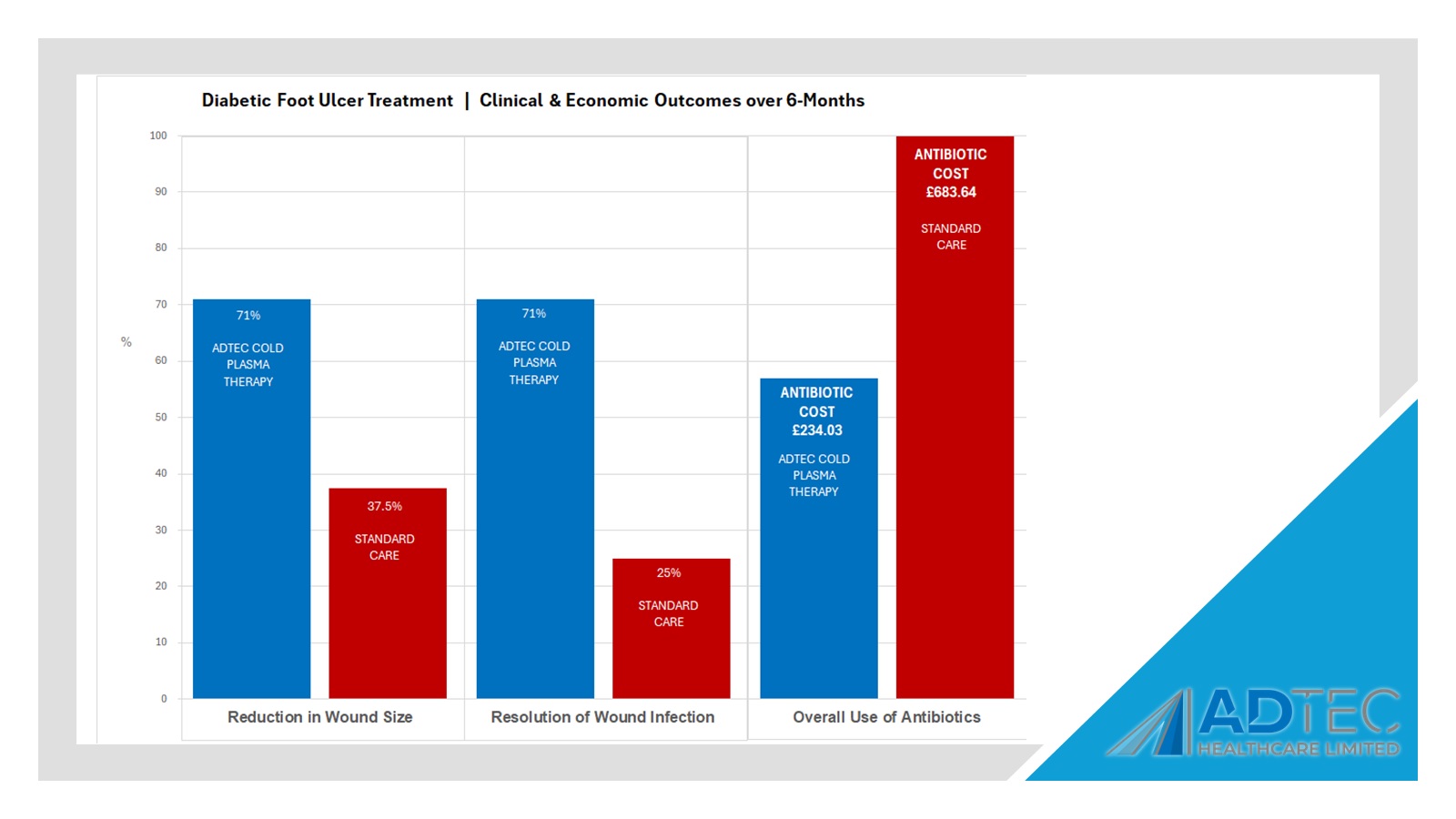
SteriPlas proven to lower antibiotic usage
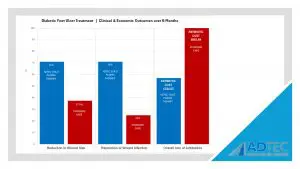
“Patients in the CAP treatment group were more likely to achieve reduction in wound size (71% vs 37.5%), resolution of wounds infection (71% vs 25%) and lower overall use of antibiotics (57% vs 100%).”
Our Health Economics data further demonstrates why the SteriPlas Premium Cold Plasma is the leading medical device for chronic diabetic foot ulcers. Continuing to relieve the cost burden associated to hospitals dealing with infection, the SteriPlas has been transforming how modern medicine works. Many users have changed their treatment guidelines to incorporate the SteriPlas and replace existing therapies that failed to work as well as the SteriPlas does.
For more information about our strong clinical efficacy and Health Economics, contact us at info@adtecplasma.com
SteriPlas to be presented at the DFSG conference
Adtec looks forward to supporting Ms Jemma Cruickshank, Antimicrobial Pharmacy Technician from Kettering General Hospital NHS Foundation Trust, at the Diabetic Foot Study Group (DFSG) conference in September.
Jemma has been invited to present the “Retrospective review of the use of argon cold plasma therapy in non-healing diabetic foot ulcers over a 3-year period within a DGH Diabetic Foot MDT Service” at the conference.
Her presentation will include the strong benefits of the SteriPlas on chronic and large diabetic foot ulcers prone to biofilm infections.
The abstract for this presentation can be found here: https://distribute.m-anage.com/from.storage?image=erIsXlaDuNOv64Tv4JyKY6XW3cI1HigkZNgT5cOgYv7Vzt_UHvGbDPesijzVroVO0
For more information about our SteriPlas Premium Cold Plasma medical device, send us an email info@adtecplasma.com
Life Science Intelligence Conference in Barcelona

Adtec Healthcare Ltd will be presenting at the Life Science Intelligence (LSI) Barcelona from 18-22 September 2023. Our Managing Director, Mary, will be showcasing the successes Adtec Healthcare and the SteriPlas have brought to clinical practices dealing with non-healing chronic wounds burdened with biofilm. Mary’s presentation will be held at 10:35 - 10:44 on September 21st and we encourage you to attend and ask your questions to better learn why we remain the leader of high density Cold Plasma medicine.
SteriPlas will be presented at the ISMCS 2023

Congratulations to Mr Thomas Schlöglhofer who has been invited to speak at the International Society for Mechanical Circulatory Support Scientific Congress (ISMCS) on Wednesday 1st November 2023.
His presentation, “Cold Atmospheric Plasma for Driveline Infection Treatment” will focus on the benefits of the SteriPlas for LVAD infections.
Our medical device has proven efficacy for the treatment of deep LVAD infections with the presence of biofilm. There is growing interest to use our SteriPlas Cold Plasma for LVAD infections due to its quick ability to destroy bacteria protected within biofilm deep within driveline cavity. This is regardless of the type of bacteria or its resistance profile. As LVAD infections can be healed in as quick as 1 week, it provides stability of the independent system and a better quality of life for patients with LVADs.
For more information about the SteriPlas, our clinical evidence or a free trial at your hospital contact us at info@adtecplasma
Thomas presents at the ISHLT show


A fantastic presentation delivered by Thomas Schlöglhofer yesterday at the ISHLT - International Society for Heart and Lung Transplantation show. We hope you got the chance to see his presentation and the promising results he has achieved managing LVAD infections with our Cold Plasma SteriPlas medical device. If you are interested to learn more about the SteriPlas and the benefits it can bring to your patients, please contact us at info@adtecplasma.com
Cold Plasma Diabetic Foot Ulcer Clinical Trial Published
Congratulations to Dr Cornelia Wiegand for her published clinical trial paper, “The efficacy of non-thermal gas plasma in the treatment of diabetic foot ulcers stalled by subclinical, biofilm-related wound infection”. This clinical trial paper features the use of our SteriPlas Cold Plasma medical device for the treatment of chronic DFUs infected with biofilm.
The parameters of this study were set much higher than a normal study, with there being a much higher standard of care given (3x per week) vs a typical outpatient setting of 1x weekly.
We wanted to show that even when chronic diabetic foot ulcers are subjected to more frequent outpatient visits with biopsies and swabs included (something not often collected during standard outpatient visits), SteriPlas Cold Plasma still prevails as the successful treatment method due to its physical mode of action and ability to kill all types of bacteria protected within biofilm.
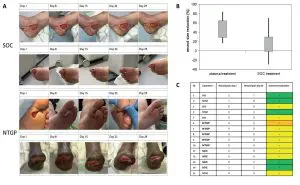
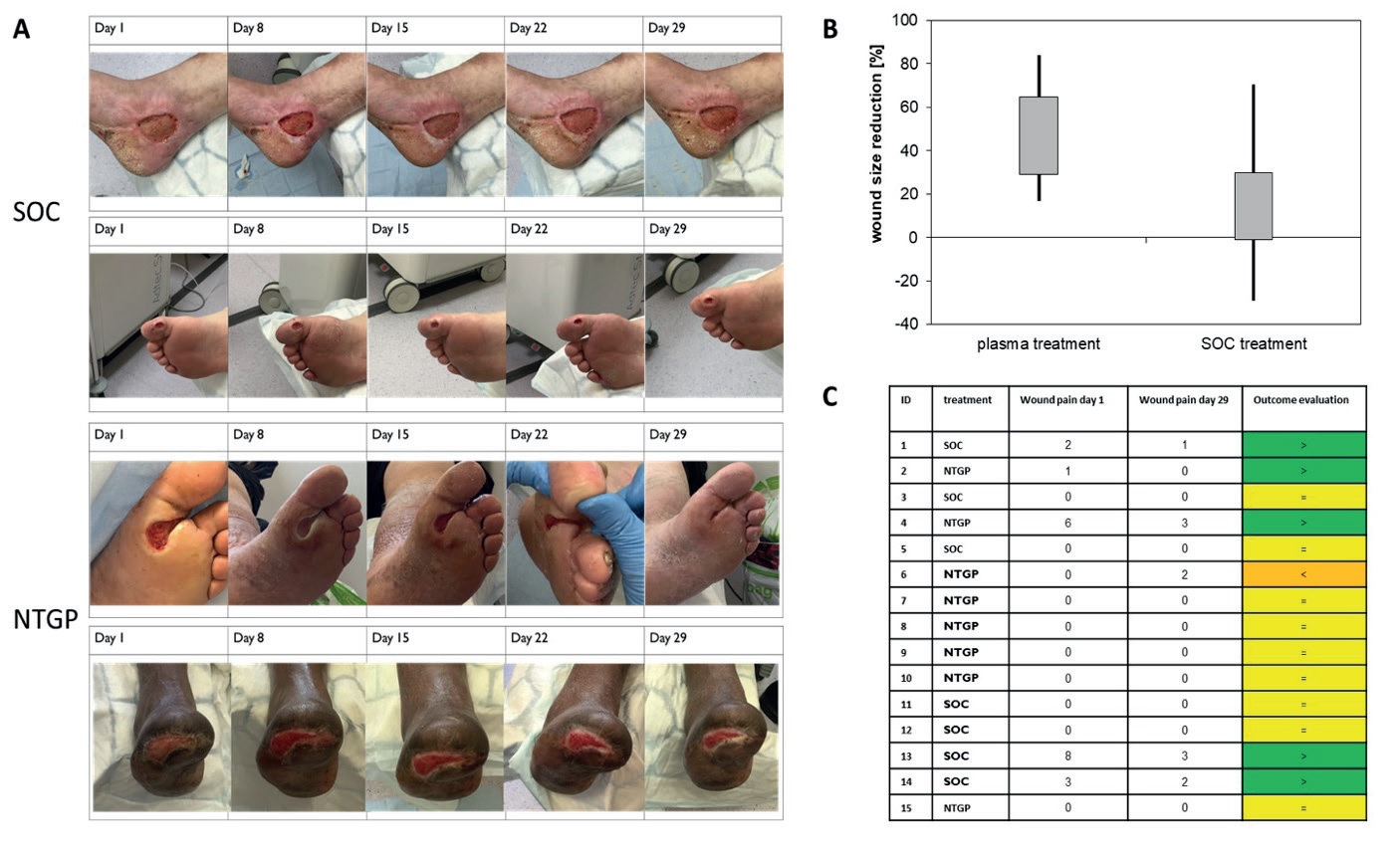
New Wound Management of Driveline Infections with Cold Atmospheric Plasma

We’re ecstatic to see Dr Jamila Kremer’s publication, “New Wound Management of Driveline Infections with Cold Atmospheric Plasma” is now live for public viewing. The paper can be viewed here: https://doi.org/10.3390/jcdd9110405.
The use of our Adtec cold plasma medical device has greatly benefitted her LVAD infection patients treated at the University Heidelberg Hospital since 2019. The results stipulate a stronger infection management advantage over conventional therapy alone for LVAD infections. This is coupled with the benefit of our cold plasma being safe, painless, contact-free and free from side effects.
Driveline infections are the most prevalent infections and reported in up to 60% of LVAD patient cases. Infection of the LVAD system is the fourth most common cause of death within one year after implantation. Dr Kremer’s publication documents Adtec cold plasma treated patients showed a 100% survival rate.
For more information about the benefits of our Cold Plasma medical device, contact us at info@adtecplasma.com