Adtec Healthcare prepares for exhibition in July

We are very excited to be exhibiting at the Malvern Diabetic Foot Conference between 7-9th July. This will be our first physical exhibition participation in over a year! Be sure to visit us and see our cold plasma medical device live in action, perfect for the treatment of chronic DFUs infected with biofilm.
Adtec SteriPlas for the treatment of deep sternal wound infections and infected drivelines
The Adtec SteriPlas has already been well documented for the treatment of deep sternal wound infections and infected drivelines. Separate studies from two leading hospitals in Germany (University Hospital Munster and University Hospital Heidelberg) have recorded the strong efficacy of our cold plasma treatment on these problematic conditions. Due to the original difficulty of managing these chronic infections using standard therapies along with the high rate of morbidity and mortality, it is no wonder why the Adtec SteriPlas has been chosen as the preferred treatment method.
You can read more about its story in the latest addition of the Chirurgische Allgemeine magazine: eSD_AdTecPlasma


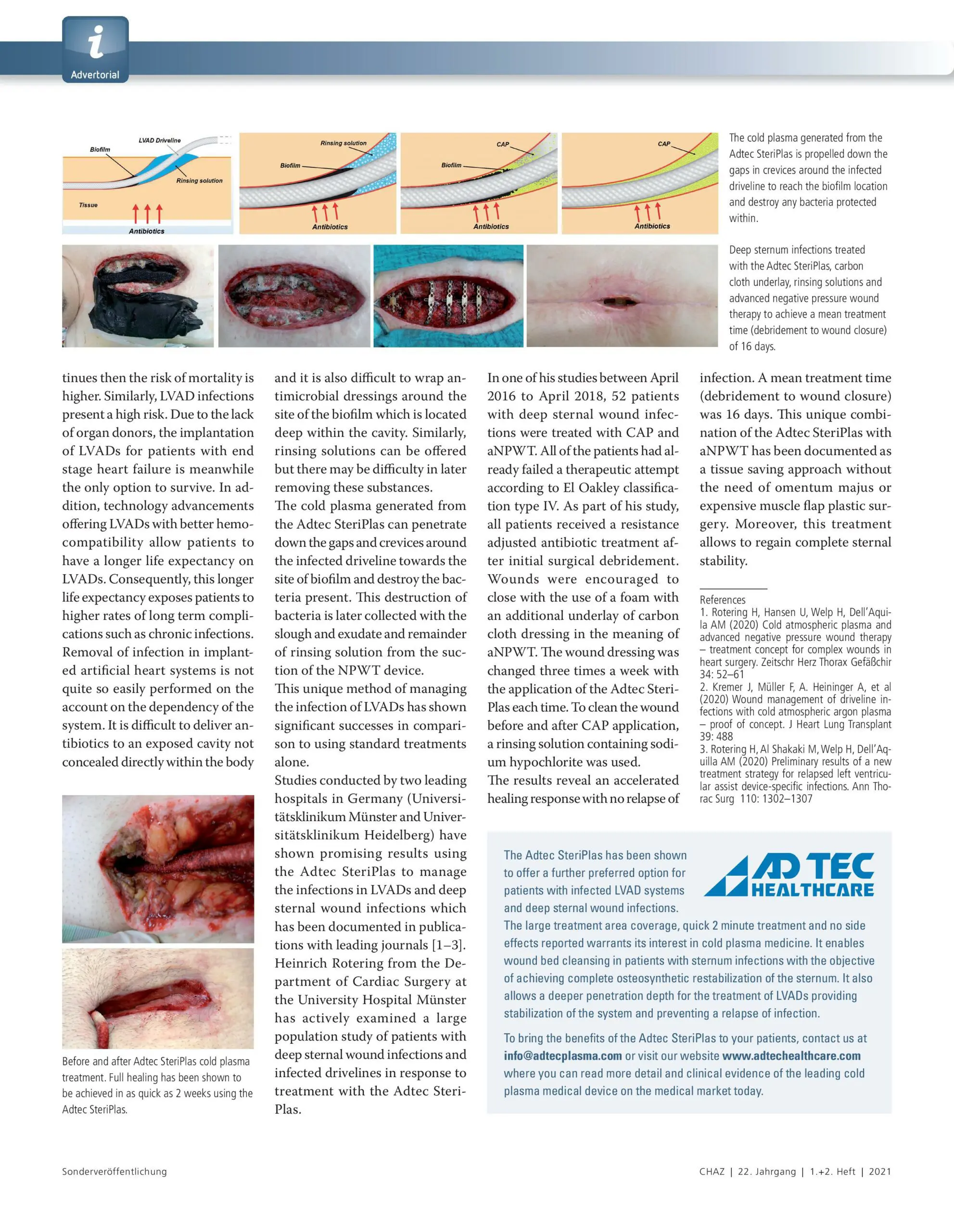
Patient and user safety is priority with the Adtec SteriPlas
As a medical device company, we always strive to ensure the priority of patient and user safety. In comparison to battery-powered and smaller cold plasma devices which may produce unreliable and damaging levels of ozone and NOx, the Adtec SteriPlas has been carefully designed as a safe and well-balanced cold plasma medical device. It has been engineered to deliver predictable levels of cold plasma to ensure the continuity of no side effects reported, putting safety and efficacy at the top priority.

New SteriPlas version and change in device Classification
Due to our proven clinical success in treating non-healing and challenging wounds, we are proud to announce the upgrade of our medical device classification to Class IIb for the Adtec SteriPlas .
This change in medical device classification also supports its continued use for treating chronic and deep wounds and surgical site infections and for treating dermatological conditions.
We also announce the product launch of a new version of our SteriPlas Version 2 model in our Cold Plasma medical device family delivering the same performance. We are very proud of the safety of our devices with no incidents or adverse events reported in 15 years of use.
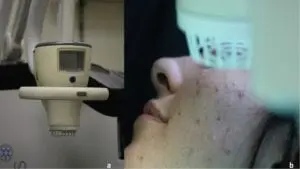
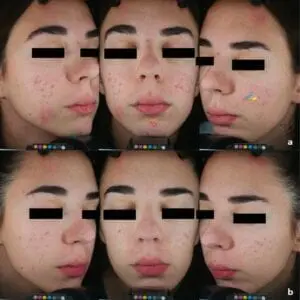
SteriPlas treatment for Acne Vulgaris
We are excited to announce the recent publication, “Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris” which features our Adtec SteriPlas cold plasma for the treatment of acne.
The paper created by Dr Mariachiara Arisi from the Department of Dermatology at the University of Brescia, Italy demonstrates the antibacterial efficacy of our patented cold plasma for the treatment of acne vulgaris patients. It indicates the significant reduction of acne skin lesions in treated patients who were previously unsuccessful treated with topical drugs. Unlike topical drugs, the Adtec SteriPlas demonstrated a safe, effective, and well-tolerated treatment option with no side effects for the treatment of acne patients.
No adverse effects or skin reactions were reported either during the treatment nor at 3-months follow-up. Treatment was completely painless and well tolerated. Patients did not report itching or burning sensation during plasma application and in the following days.
The full paper can be found here: https://www.sciencedirect.com/science/article/pii/S2212816620300172
Leading cold plasma medical device
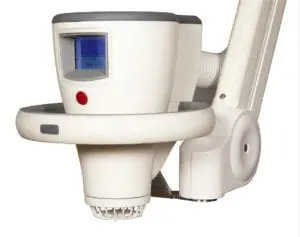
The Adtec SteriPlas is the leading cold plasma medical device used to manage the infection in chronic wounds, surgical site infections and to treat dermatological conditions such as acne and actinic keratoses.
With our wide clinical trials and publications bibliography, we are proud to state its strong antibacterial efficacy regardless of the type of bacteria or its resistance profile. There have been no side effects reported with the use of our medical device since it is safe, painless to touch and an effective method of treating patients from conventional therapies.
Adtec Healthcare exhibiting at two leading wound conferences this year
Adtec Healthcare looks forward to exhibit at the European Wound Management Association (EWMA) conference and The Malvern Diabetic Foot Conference in May 2020. As the two conferences will be running on the same dates (13th-15th May ’20), we will have teams exhibiting at both conferences so we can meet with you at whichever conference you attend.
We will have our medical device live on display at both exhibitions and look forward to inviting you to our booth to give a demonstration on its ease of use to achieve remarkable results.


#EWMA #EWMA2020 #MalvernDFU #Malvern2020 #wound #wundkongress #coldplasma #kaltesplasma #gasplasma #plasma #exhibition #woundcare #surgicalsiteinfection #preventSSI #SSIprevention
Randomised Controlled Clinical Trials of Adtec SteriPlas/MicroPlaSter Plasma Treatment of Wounds and Dermatological conditions over a 14 year period.
Adtec Healthcare’s plasma technology has been tested in 7 randomised controlled clinical trials with proven clinical efficacy and safety. The first participant in an RCT with our plasma technology was on 24th October 2005 - This was also the first RCT worldwide to investigate the clinical efficacy of cold plasma. We continue to be actively involved in RCTs and clinical studies with SteriPlas to produce strong clinical evidence.
The results of these trials include wound size reduction, bigger reduction of bacterial load compared to antibiotics, treatment of AK lesions and reduction of pain with no adverse events reported.
1. ACTICAP - A prospective, randomised, monocentric, rater blinded study evaluating the change of the cutaneous microbiome in correlation to the efficacy of cold atmospheric plasma treatment in comparison to diclofenac 3% in 2.5% hyaluronic acid (Solaraze 3% Gel®) in patients with actinic keratoses
University Hospital Essen, Germany
Status – Trial completed 2019 and publication planned 2020
2. Prospective, Randomised and Placebo Controlled Clinical Trial (RCT) for the validation of treatment of chronic wounds with cold atmospheric plasma (CAP)
University Hospital Essen, Germany
Status – Trial completed 2018 and publication planned 2020
3. A randomised controlled trial to evaluate the efficacy of non-thermal gas plasma (NTGP) on sub-clinical wound infection (biofilm) in patients with diabetic foot ulcers compared to those treated with standard of care dressings
Salford Royal Foundation Trust, UK
Leeds TeachingHospital, UK
Status – Trial ongoing
4. Low temperature argon plasma for in vivo sterilization of chronic wounds
Klinikum Schwabing, Germany
University Hospital Regensburg Germany
Status – Trial completed 2013 and published
Successful and Safe Use of 2 Min Cold Atmospheric Argon Plasma in Chronic Wounds: Results of A Randomized Controlled Trial. Isbary ,British Journal of Dermatology, 2012.
A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients, Isbary ,British Journal of Dermatology, 2010
5. Randomised placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites
University Hospital Regensburg Germany
Status – Trial completed and published
Randomized placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites. Heinlin Wound Repair Reg 2013
6. Randomised placebo-controlled clinical trial showed cold atmospheric
argon plasma in herpes zoster
Klinikum Schwabing, Germany
University Hospital Regensburg Germany
Status – Trial completed and published
Randomized placebo-controlled clinical trial showed cold atmospheric argon `plasma relieved acute pain and accelerated healing in herpes zoster, Isbary , Clinical Plasma Medicine,2014,
7. A randomised two-sided placebo-controlled study on the efficacy and safety of atmospheric non-thermal argon plasma for pruritus
Klinikum Schwabing, Germany
University Hospital Regensburg Germany
Status – Trial completed and published
A randomized two-sided placebo-controlled study on the efficacy and safety of atmospheric non-thermal argon plasma for pruritus, Heinlin, J Eur Acad Dermatol Venereol. 2013.
Adtec Plasma in CSP Renewable Energy Project

Adtec Europe Ltd is sponsoring two post-graduate students to do research in new potential applications for plasma in collaboration with Cranfield University.
The focus of this research is to understand the utilisation of plasma-assisted surface conditioning of low-iron glass solar reflecting mirrors for concentrating solar thermal power applications. The research project is supervised by leading experts in this field Professor Chris Sansom and Dr Peter King of Cranfield University and Dr Adam Bennett of Cranfield Plasma Solutions.
CSP plants generate electricity by concentrating sun light with large arrays of mirrors which are usually located in desert regions. Consequently, the mirrors get covered in sand and dust, and require cleaning with brushes and water on a regular basis. Currently much water is used to clean the mirrors, a precious resource in arid terrains. The aim of this project is to investigate the characteristics of a novel atmospheric pressure plasma system used to condition CSP concentrating mirrors which will be capable of reducing the amount of water used in the cleaning process.
The Global Concentrating Solar Power (CSP) market was valued at over $3 Billion US in 2016 and is anticipated to grow by 13% by 2025. There is also a compelling business desire to undertake this project. The development of a novel atmospheric pressure plasma system will be a significant game changer in the CSP market. Such a disruptive technology is anticipated to yield significant commercial benefits.

#CSP #concentratingsolarpower #solarpower #solar #solarenergy #renewableenergy #greenenergy #energy #cleanenergy #sustainableenergy #sustainablepower #sustainableenvironment #plasma #gasplasma #coldplasma #kaltesplasma #airplasma
EWMA
We had a great time exhibiting at the EWMA 2019 conference. We would like to congratulate Maurice Moelleken, Dr Heinrich Rotering and Dr Michael Pierides for their brilliant presentations during the conference. Their data showed the strong benefits of using the Adtec SteriPlas on chronic wounds and surgical site infections, offering an alternative to standard treatment therapies that bacteria may pose a resistance to.
The Adtec SteriPlas has proven antibacterial efficacy backed by a wide clinical bibliography and no side effects reported making it safe, painless and effective for the treatment of infected wounds stalled by bacteria.
For more information send us an email to info@adtec.eu.com


#EWMA #EWMA2019 #antimicrobialresistance #medicaldevice
#gasplasma #coldplasma #kaltesplasma