Life Science Intelligence Conference in Barcelona

Adtec Healthcare Ltd will be presenting at the Life Science Intelligence (LSI) Barcelona from 18-22 September 2023. Our Managing Director, Mary, will be showcasing the successes Adtec Healthcare and the SteriPlas have brought to clinical practices dealing with non-healing chronic wounds burdened with biofilm. Mary’s presentation will be held at 10:35 - 10:44 on September 21st and we encourage you to attend and ask your questions to better learn why we remain the leader of high density Cold Plasma medicine.
Advanced Wound Care Summit 2023


We hope you got to see our presentation at the Advanced Wound Care Summit yesterday. We thoroughly enjoyed explaining the strong benefits of our SteriPlas Cold Plasma for biofilm destruction. If you would like to learn more about our medical device and clinical evidence, send us an email info@adtecplasma.com
Size Does Matter!

Size does matter!
Yes, we do manufacture smaller handheld Cold Plasma devices that have antibacterial efficacy but we do not sell these in the medical field. A quick 2 minute treatment time is respective of being focused on the size of the plasma area created. Small and handheld plasma jet devices feature a 1mm2 treatment area which means antibacterial efficacy is achieved in a very small area for every 2 minutes applied. If the wound is 10cm2, we will leave you to do the maths on how long it will take to treat! This is why we do not sell handheld plasma jet pens as medical devices as they are limited to small and superficial wounds.
The SteriPlas creates a gigantic 12cm2 treatment area which is only possible through the size of the medical device which incorporates a series of crucial components such as the generator and control unit. This means the SteriPlas can cover large wounds and finish treatment in as quick as a single two minute session. Whilst the SteriPlas can treat acute and superficial wounds, it is designed to target deep and complicated wounds with #biofilm.
Deep sternal wounds is a classic example as documented in our clinical evidence.
The Leader of Cold Plasma
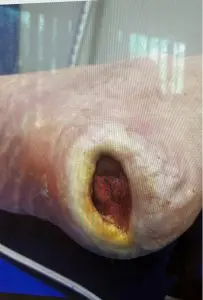
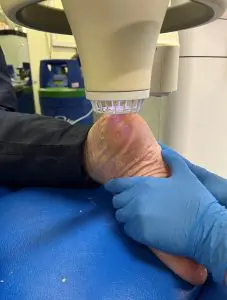
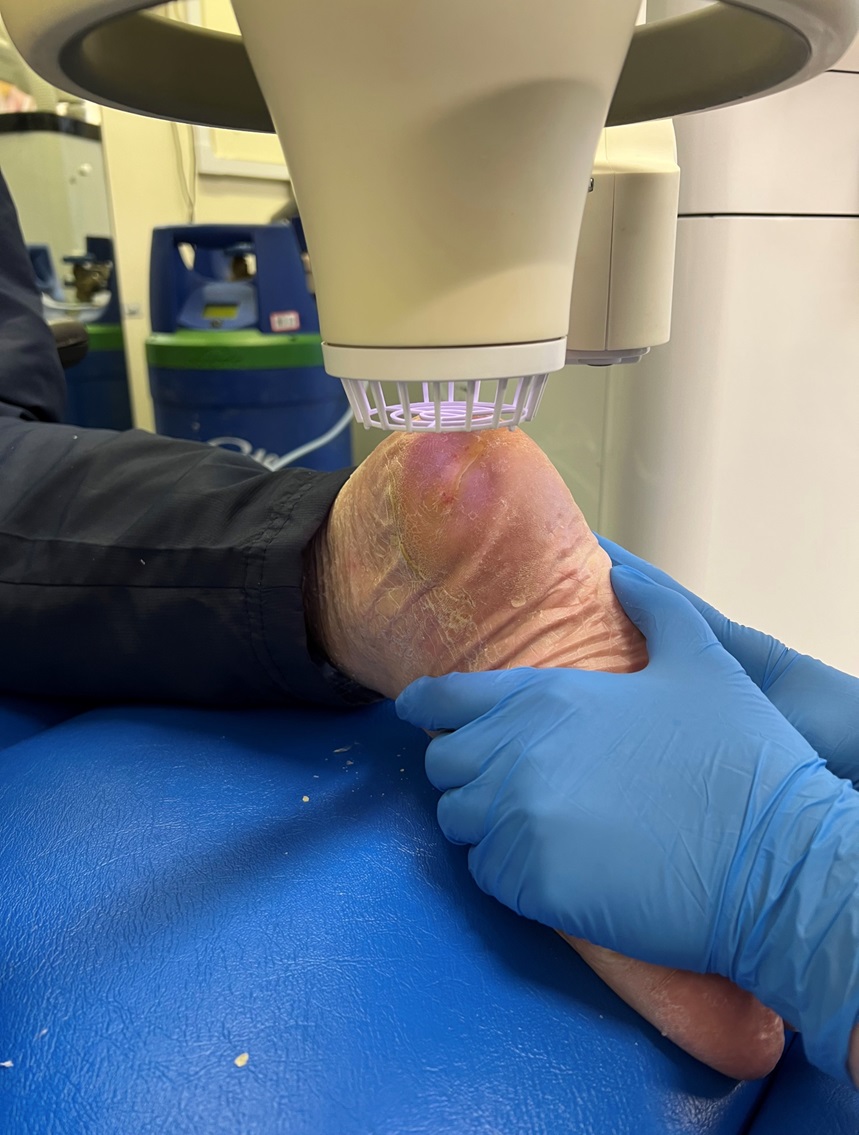
There is expanding interest of our medical device for the treatment of chronic diabetic foot ulcers, deep sternal wounds and LVAD infections. We wish to remind you that if you are a Health Care Professional seeking to learn more about our medical device then please do get in contact with us. A member of our team will be able to show the full benefits and safety of using our medical device to treat these complicated conditions, with no side effects.
As Adtec Healthcare remains the leader of cold plasma medicine, we are lucky to be able to boast a wide clinical bibliography collection that really makes us stand out amongst the rest. We were the first company worldwide to do clinical trials of cold plasma on chronic wounds. Our clinical trials data has paved way in today’s medicine to show cold plasma as the go-to alternative for infection management.
If you are interested to bring the benefits of cold plasma to your patients, please email us at info@adtecplasma.com
Cold Plasma Walk at ISHLT
We’re excited to be exhibiting at the ISHLT conference next week and we look forward to supporting Mr Thomas Schlöglhofer as he presents his SteriPlas Cold Plasma presentation for the management of LVAD infections during the “Bugs, Drugs, and Thugs: Fighting Infection in VADs Any Way We Can” session on Wednesday 19th April between 5:30 PM - 6:30 PM.
Due to popular demand, we will be hosting a Cold Plasma Walk session at our exhibition booth on Wednesday 19th April during the lunch break. We encourage you to visit our booth no. 416 so you can see Thomas present our medical device and explain the strong benefits it can bring to the management of LVAD infections.
Our proven and patented Cold Plasma medical device has been widely adopted as the solution for LVAD infections due to its unique physical mode of action, quick treatment times and accelerated healing with stalled infections due to biofilm all whilst protecting the patient and user from no side effects.
Contact us at info@adtecplasma.com for more information.
Day 2 of the SCTS

It's Day 2 of the Society for Cardiothoracic Surgery in GB & Ireland (SCTS) conference and we've been overwhelmed with all your interest in our Cold Plasma medical device, the SteriPlas. Visit us at booth 18 to learn more about how to bring the benefits of our medical device to your hospital.
SteriPlas heals wounds quickly

Did you catch the leading Serbian media newspaper, Večernje Novosti, last week? Our SteriPlas was featured in the article, “Cold plasma heals wounds quickly” composed by Dr Zorić.
Dr Zoric has been using our cold plasma medical device to treat hard-to-heal and stalled wounds infected with biofilm. These problematic wounds have responded very well with our SteriPlas treatment and patients have been healed that were otherwise stalled by conventional therapies.
If you are within Serbia and interested to learn more about our medical device, contact our medical distributor Borf Health Care on info@borf.biz for more information.
Invited to speak at the ICCAC meeting on 10th January
We are honoured to be invited to speak at The International Consortium of Circulatory Assist Clinicians (ICCAC) meeting tomorrow. Our presentation will focus on the strong benefits of the Adtec SteriPlas cold plasma for the management and healing of LVAD infections. With growing interest for an alternative therapy to failing antibiotics, the SteriPlas has been storming the medical market because of its accelerated healing, safe, effective, and harmless properties all of which will be discussed tomorrow at 3.30pm (CET).
Please contact us at info@adtecplasma.com for more information.
Presentation at the DFSG 2022
We're so excited for exhibiting at the Diabetic Foot Study Group (DFSG) conference next week. Please be sure to visit us at booth no.11 where we will based during the conference. We welcome you to see our SteriPlas Cold Plasma medical device live in action.
We're also excited to announce that the Diabetes Team at Kettering General Hospital NHS Foundation Trust will have a poster presentation illustrating the strong antibacterial efficacy and healing qualities of the Adtec SteriPlas for diabetic foot ulcers.
The poster, "DGH EXPERIENCE OF USING COLD PLASMA MEDICAL DEVICE AS AN ADJUNCT THERAPY TO ANTIMICROBIALS IN TREATING CHRONIC NON HEALING INFECTED DIABETIC FOOT ULCERS" will be presented by Jemma Cruickshank on Saturday 17th September, 13:25 - 14:25.
See you all soon!
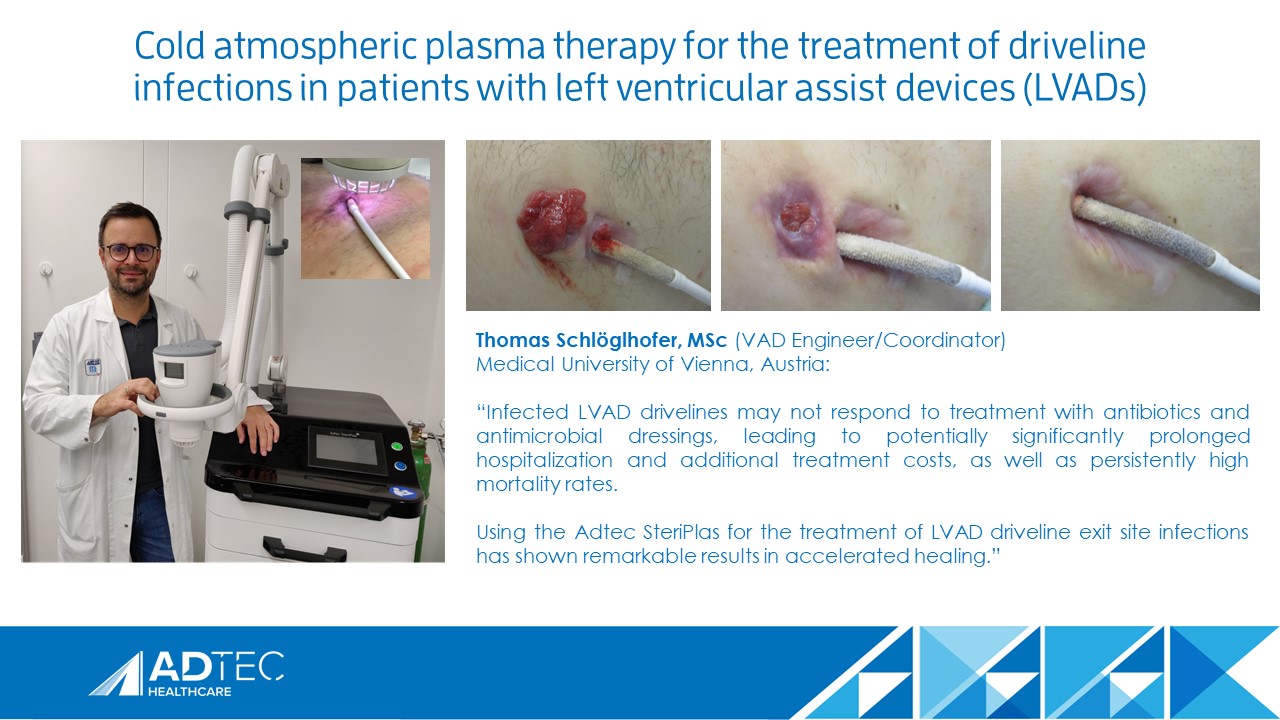
The ISHLT discusses the Cold Plasma Project
We’re ecstatic to see our Adtec SteriPlas continue to raise awareness in the LVAD community. A great interview between the ISHLT and Mr Thomas Schlöglhofer from the Medical University of Vienna discussing the benefits of our cold atmospheric plasma medical device for the treatment of LVAD Infections and their broad study being conducted.
Thomas has been an avid user of our medical device for some time now collecting remarkable results with his LVAD patients, documenting the strong benefits of our medical device including the significant reduction of infection rates coupled with full healing, reduction of readmittance and the stabilisation of LVAD systems all with the added benefit of no side effects.
We look forward to the completion of this study and the publishing of the data which will be discussed at the ISHLT 2023 conference next year. We will also be exhibiting at this conference and excited to meet more people from the LVAD community.
https://youtu.be/6-L0sgXLx9o