The Science Behind SteriPlas Cold Plasma
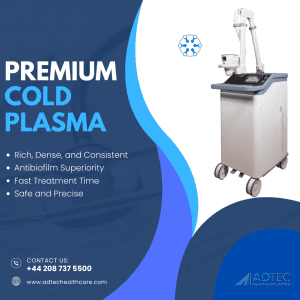
What makes the SteriPlas Premium Cold Plasma medical device so powerful in chronic wound care? It's all about cutting-edge technology. We use microwave 2.4GHz technology to generate argon cold plasma, and here’s why this combination is unmatched:
✅ Rich, Dense, and Consistent Cold Plasma: Delivers a premium plasma field for reliable and effective wound care treatments.
✅ Antibiofilm Superiority: Effectively breaks down bacteria protected within biofilms that block healing and resist traditional treatments.
✅ Fast Treatment Time: Just 2 minutes per 12cm² to achieve antibacterial effectiveness—saving time without compromising results.
✅ Safe and Precise: Targeted application with no side effects for patients.
This ideal combination of microwave technology and argon cold plasma ensures superior performance for chronic infection management and wound healing.
We're redefining care with science that heals.
Redefining Global Infection Care
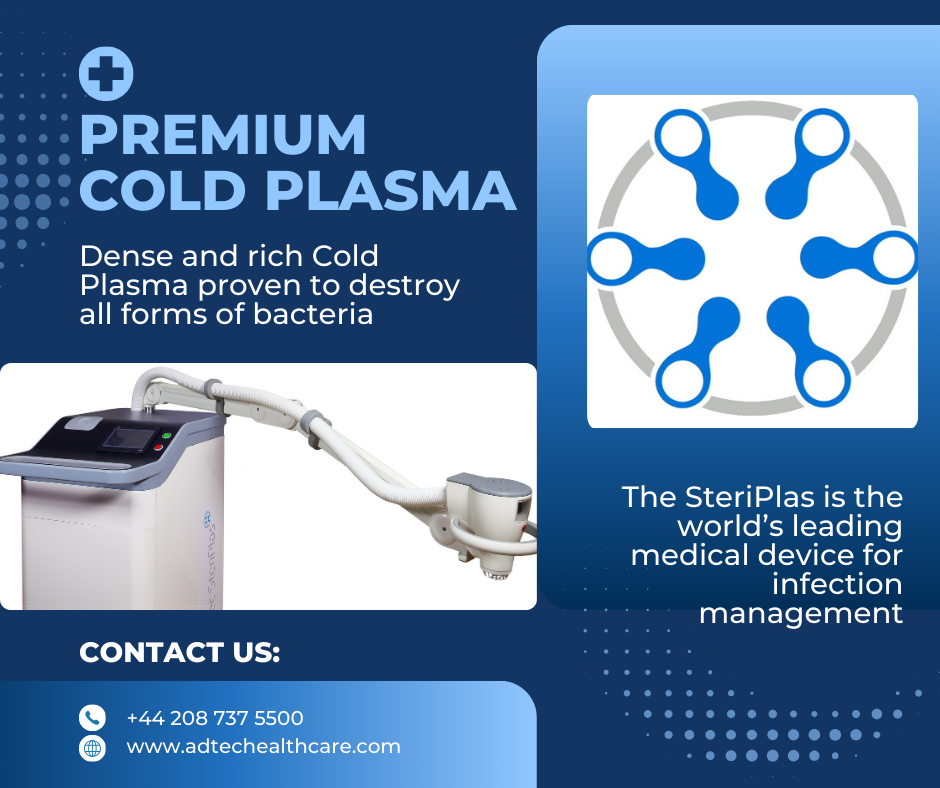
Changing Lives with the SteriPlas Cold Plasma
At the forefront of advanced wound care, our SteriPlas Premium Cold Plasma medical device offers unparalleled benefits for clinicians and patients alike. Here's how we're driving innovation:
✅ Broad Wound Treatment Capability: Effective for diabetic foot ulcers, burns, surgical wounds, autoimmune-related wounds, and more.
✅ Biofilm Eradication: Breaks down stubborn bacteria protected within biofilms that prevent healing—a key advantage in chronic infection care.
✅ Drug-Free Alternative: Reduces or eliminates the need for antibiotics, supporting global antimicrobial stewardship efforts.
✅ Portable and User-Friendly: Designed for ease of use in busy hospital settings or outpatient clinics.
✅ Scientifically Proven: Backed by strong clinical evidence and microwave argon technology to deliver consistently positive patient outcomes.
The SteriPlas is redefining what’s possible in wound management, giving patients renewed hope for recovery.
Together, let’s heal better.
Elevating Wound Care with SteriPlas Cold Plasma

Elevating Wound Care with SteriPlas Cold Plasma
Our SteriPlas Premium Cold Plasma medical device with powerful microwave argon technology continues to transform chronic wound management with innovative features that make a real difference for patients and healthcare professionals alike.
Here’s what sets the SteriPlas apart:
✅ Pain-Free Application: Gentle yet highly effective, the SteriPlas delivers treatment without discomfort for patients.
✅ Non-Invasive Therapy: No need for surgical interventions or harmful chemicals—just clean, advanced plasma science.
✅ Fast Treatment Times: Delivers powerful results with quick, convenient sessions that fit seamlessly into clinical workflows.
✅ Antibacterial Power: Effectively eliminates all forms of bacteria including superbugs.
By offering a safe, effective, and cutting-edge approach to chronic wound care, the SteriPlas is empowering clinicians to achieve better outcomes for their patients.
Revolutionising global Infection Care
Revolutionizing Wound Care with SteriPlas Premium Cold Plasma
When it comes to chronic wound infections, our SteriPlas Premium Cold Plasma medical device is setting the gold standard in advanced care. Here's why healthcare professionals are choosing SteriPlas:
✅ Dense, Premium-Rich Cold Plasma: Our advanced microwave argon technology generates a superior cold plasma that penetrates deeper and delivers a more powerful therapeutic effect than competitor devices.
✅ Biofilm Breakthrough: Effectively tackles biofilm complications, a leading cause of stalled healing in chronic infections.
✅ Versatility: Proven effective across a wide range of wound types—including diabetic foot ulcers, LVAD infections, burns, and post-surgical wounds.
✅ No Side Effects: Gentle yet powerful, patients experience no adverse reactions.
With SteriPlas, healthcare professionals are redefining what's possible for patients with wounds that resist traditional treatments.
Together, we’re transforming the future of chronic wound care.
Why Size Matters

Why Size Matters in Cold Plasma Therapy: How SteriPlas Redefines Safety and Efficacy in Wound Treatment
In the fast-evolving world of medical technology, size often seems to shrink in tandem with innovation. Smaller, more portable devices flood the market, promising convenience and versatility, but sometimes critical aspects of functionality can be compromised. Adtec Healthcare’s SteriPlas, a groundbreaking microwave cold plasma device for wound treatment, boldly defies this trend, emphasizing that when it comes to effective and safe medical treatment, bigger truly is better.
SteriPlas: Leading with Power, Safety, and Precision
At a time when competitors are striving for compactness, the SteriPlas stands out with its larger design, which is purposefully engineered to deliver an unmatched level of therapeutic cold plasma. Unlike smaller Cold Plasma devices, the SteriPlas was built to prioritize treatment potency and safety without compromising on effectiveness. But why exactly does size matter for the SteriPlas? The answer lies in its ability to generate a rich, dense cold microwave plasma field that smaller devices simply can’t match.
Delivering Dense Cold Plasma for Optimal Therapeutic Effect
Cold plasma, a unique, non-thermal form of energized gas, has become a significant breakthrough in wound care due to its ability to kill bacteria. However, the efficacy of cold plasma therapy hinges on both the density and consistency of the plasma field, which is directly impacted by the device’s design.
The SteriPlas, with its larger structure, is engineered to generate and sustain a denser, richer microwave argon plasma field. This dense field ensures that cold plasma thoroughly reaches the treatment area, making it more effective at eradicating harmful pathogens and promoting healing. Where smaller devices might struggle to deliver a consistent plasma , the SteriPlas provides a comprehensive, evenly distributed cold plasma field, amplifying its therapeutic benefits.
Prioritizing Safety with No Side Effects
The SteriPlas was also designed with patient safety as a top priority. Despite its larger size and higher plasma density, it remains exceptionally safe for patients, no reported side effects—a critical advantage in any therapeutic device. Smaller devices may sometimes trade off treatment efficacy to meet compact design requirements, but the SteriPlas’s size is integral to its uncompromising safety standards.
Cold plasma therapy’s expanding use in wound treatment has led to a surge in demand for devices that are not only effective but also safe for prolonged and repeated use. SteriPlas’s larger design enables it to incorporate advanced safety protocols that allow it to maintain a rich microwave plasma field without risking tissue damage or other side effects. This design ensures that the SteriPlas can safely treat sensitive areas and deliver results consistently over time.
Setting a New Standard in Cold Plasma Therapy
The SteriPlas represents a thoughtful balance of innovation, efficacy, and patient-centered design, challenging the notion that smaller is always better. While compact devices may offer some portability, the SteriPlas delivers a level of cold plasma density and therapeutic power that smaller devices cannot replicate. This enhanced efficacy, combined with its rigorous safety measures, positions SteriPlas as an ideal choice for clinics and healthcare providers seeking the best outcomes for their patients.
In wound care, where every treatment counts, the SteriPlas’s larger design is not just a feature—it’s a necessity. It’s a testament to Adtec Healthcare’s commitment to ensuring that patients receive the highest standard of care without compromise.
Conclusion
As cold plasma therapy continues to grow in its applications, healthcare professionals face an important decision in choosing devices that prioritize both treatment potency and patient safety. With its robust, thoughtfully engineered design, the SteriPlas offers an unparalleled therapeutic solution that addresses both needs, setting a new benchmark in cold plasma wound therapy. Adtec Healthcare invites clinicians to explore the SteriPlas difference, where bigger means better, safer, and more effective healing.
SteriPlas combats Antimicrobial Resistance (AMR)

In 2016, the NHS identified the need to find clinically and cost-effective interventions for patients with diabetic foot disease as a high priority (College of Podiatry, 2019). This urgency is compounded by the rapidly rising incidence of antimicrobial resistance (AMR), a particular concern in people with DFU as infections are the leading cause of amputation (Hurlow et al 2018).
The SteriPlas has the ability to kill all forms of bacteria regardless if they are Gram-positive or Gram-negative or if they are protected within biofilm. It is a broad spectrum, topical antibacterial medical device that delivers consistent Premium Cold Plasma therapy directly at the site of infection. By eliminating bacteria that prevents the body from healing, it significantly reduces the antimicrobial resistance rates and prevents amputations from occurring.
Our proven clinical trials and studies have placed the SteriPlas as the leading Cold Plasma medical device in the market.
For more information about our strong clinical efficacy and #HealthEconomics, contact us at info@adtecplasma.com
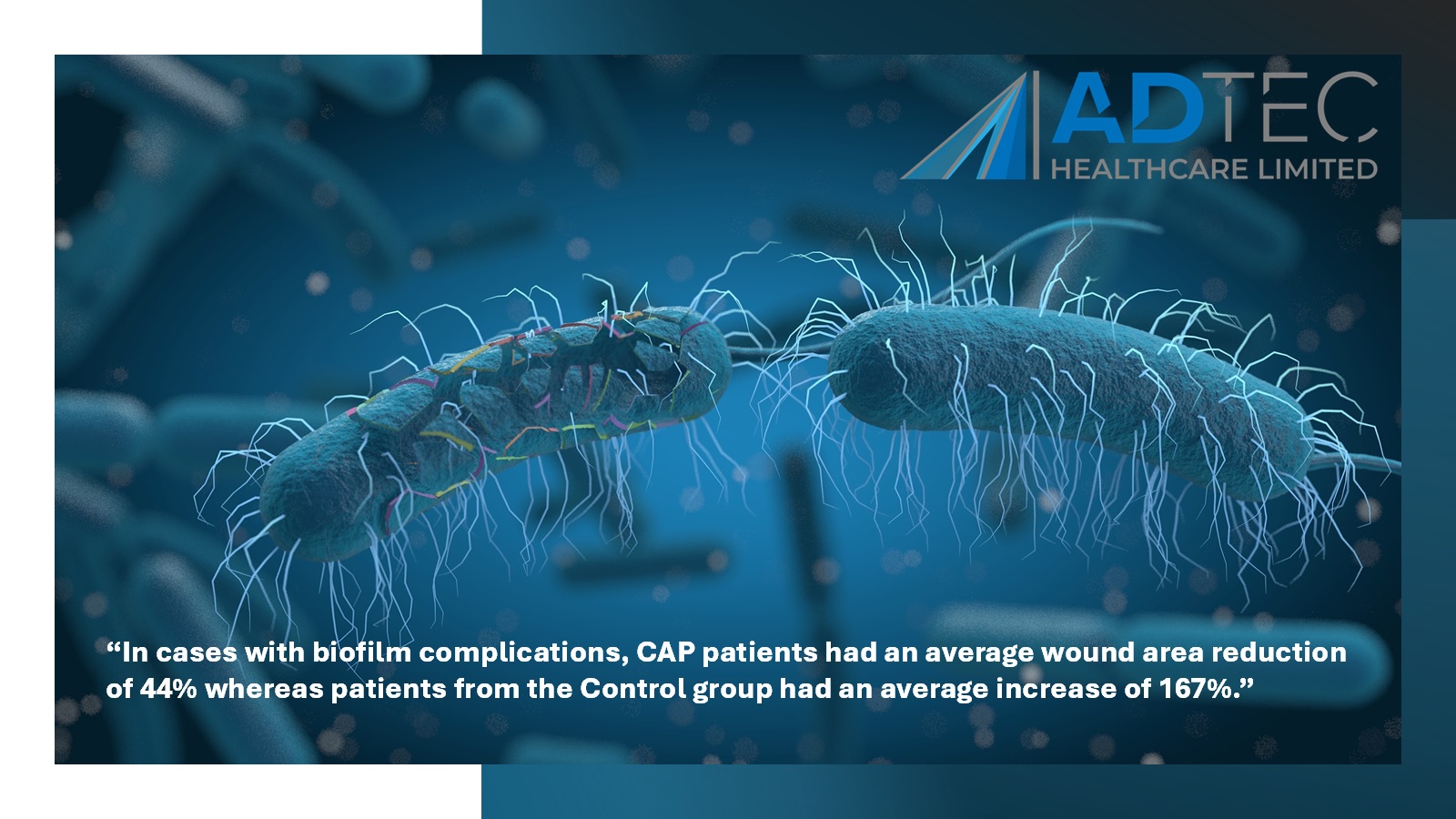
SteriPlas proven to heal patients faster
“In cases with biofilm complications, CAP patients had an average wound area reduction of 44% whereas patients from the Control group had an average increase of 167%.”
Diabetic foot ulcers complicated with biofilm are already prone to poor arterial flow, therefore, the delivery of antibiotics to a complicated site already infected with multi-resistant bacteria may pose little to no help in healing the wound. This unfortunately can lead to amputations and further complications.
The SteriPlas benefits significantly to antibiotics due to its unfailing physical mode of action delivery during treatment. Multi-resistant bacteria, whether embedded and protected in biofilm, is quickly destroyed in as quick as 2 minutes. The treatment is delivered directly at the site of infection, is painless, contact-free, and most importantly has no side effects. As bacteria is destroyed so easily with our Premium Cold Plasma therapy, patients are healed and relieve the costs burden associated to hospitals.
“The SteriPlas is a cost and clinically effective treatment option for diabetic patients with infected chronic lower leg wounds in the absence of significant peripheral arterial disease.”
For more information about our strong clinical efficacy and #HealthEconomics, contact us at info@adtecplasma.com
Diabetic Foot Ulcer Health Economics

Adtec Healthcare Limited has invested over 10 years in clinical trials and studies to bring you over 80+ peer reviewed studies to validate why the SteriPlas Premium Cold Plasma is the leading medical device in the medical industry for chronic infection management. Not only is our medical device clearly proven to be the strongest against multi-resistant bacteria protected within biofilm but it can treat a wide variety of deep and hard to reach areas that are often recalcitrant to conventional therapies, therefore not limited to superficial wound treatments.
Our recent goal has been to collect the cost effectiveness findings to demonstrate just how the cost burden can be eliminated by the hospital when using our SteriPlas. In the case of chronic diabetic foot ulcers with biofilm presence in the NHS, the SteriPlas has been shown to reduce antibiotic usage by 57% for patients thus relieving the ongoing costs thrown onto the hospital service. Not only are patients healed significantly faster, bed occupancy significantly reduced, and limbs are saved but there is a significant reduction in patient visits to the hospitals thus relieving further costs associated to the hospital.
For more information about our Health Economics data, contact us at info@adtecplasma.com
What makes our SteriPlas safe and effective?

What makes our SteriPlas Premium Cold Plasma safe and effective?
Adtec has been the global leader of Cold Plasma medicine, having developed Cold Plasma products for over 20 years and being the first company worldwide to conduct clinical trials on wounds. In total, Adtec boasts over 80+ clinical trials and studies with no side effects reported.
Whilst there are many forms of Cold Plasmas on the market, our SteriPlas Cold Plasma is dense and slowly propelled towards the treatment site enabling bacterial destruction in deep and hard to reach infection areas. The argon Cold Plasma is microwave, 2.45GHz generated using crucial components within the body of the medical device. This is why it is larger in size but at the advantage of delivering reliable and consistent results and easily tackles complicated biofilm issues within chronic infections unlike the other variations of Cold Plasma on the market. This is also why our clinical evidence is often cited by other Cold Plasma manufacturers as our promising results far outweigh the results achieved by Cold Plasma devices with different plasma designs.
To learn more about our strengths and benefits, contact us at info@adtecplasma.com
Leading the way in Cold Plasma Medicine
The antibiofilm properties of the SteriPlas has been widely documented across our 80+ clinical trials and studies. It is being widely used to treat a multitude of infection conditions such as Diabetic Foot Ulcers, Sternal Wounds and LVAD infections in the UK, EU and SE Asia. Helping to significantly reduce morbidity, mortality, amputation and bed occupancy rates, the SteriPlas has been transforming modern medicine by simplifying and accelerating the way these chronic and non-healing wounds are treated. We have also documented the significant cost saving benefits of using the SteriPlas Premium Cold Plasma all with the added benefit of no side effects due to its safe, microwave 2.45GHz argon plasma generation.
To learn more about the benefits we can bring you, contact us at info@adtecplasma.com