Antimicrobial (antibiotic) Resistance – the drugs won’t work !
Antimicrobial resistance (AMR) poses a significant global threat of far-reaching proportions. It is estimated that drug resistant infections contribute to nearly 5 million deaths every year and predicted to increase to over 10 million deaths every year. WHO has declared that AMR is one of the top 10 global public health threats facing humanity.
(The worldwide total deaths from COVID is now just over 6 million and we all know how frightening it was before the vaccine was developed)
AMR occurs when bacteria, viruses, fungi and parasites change over time and no longer respond to medicines making infections harder to treat and increasing the risk of disease spread, severe illness and death. As a result, the medicines become ineffective and infections persist in the body, increasing the risk of spread to others. Microorganisms that develop antimicrobial resistance are sometimes referred to as “superbugs”. Without effective antimicrobials, the success of modern medicine in treating infections, including during major surgery and cancer chemotherapy, would be at increased risk. Chronic infections if left untreated could result in tissue damage, amputation, longer stays in hospitals, surgical interventions, or increased possibility of mortality. Patients who are infected with drug-resistant infections are more likely to develop complications and are up to three times more likely to die from the infection. Non-healing wounds in particular, are characterised by complex and mixed bacterial populations, often involving antibiotic-resistant bacteria as well as phenotypically tolerant bacteria in biofilm form. The biofilm factor is clearly of considerable clinical importance: it protects bacteria from antimicrobial agents leading to persistent and difficult to treat chronic infections, and it exacerbates the spread of antibiotic resistance. Surgical Site Infections are also linked to anti-microbial resistance.
SteriPlas cold plasma technology kills bacteria by a physical mode of action and bacteria are therefore unlikely to develop primary or secondary resistance, which we have documented from our clinical studies. SteriPlas cold plasma also kills antibiotic resistant bacteria (e.g. MRSA) and kills bacteria encased in biofilm which are typically up to 1000 times more resistant to antibiotics. SteriPlas has proven clinical efficacy in treating wound infections, diabetic foot infections and surgical site infections in all clinical studies, all with the bonus of no side effects reported.
SteriPlas cold plasma can be used to treat topical infections reserving antibiotics for severe systemic infections.
References
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02724-0/fulltext
https://www.worldometers.info/coronavirus/
https://www.who.int/health-topics/antimicrobial-resistance
https://healthfirsteurope.eu/wp-content/uploads/2020/11/A3A4-48pp-Booklet-Spreads-1.pdf
Bowler, P., Murphy, C. & Wolcott, R. Biofilm exacerbates antibiotic resistance: Is this a current oversight in antimicrobial stewardship?. Antimicrob Resist Infect Control 9, 162 (2020).
The first cold plasma medical device to treat Scleroderma

We congratulate Dr Stephanie Ardnt and Prof. Sigrid Karrer from the University Hospital Regensburg for their recent publication, “The Anti-Fibrotic Effect of Cold Atmospheric Plasma on Localized Scleroderma In Vitro and In Vivo”.
This publication features the use of our Adtec SteriPlas and shows strong efficacy for the treatment of Scleroderma, which up to now has not yet been evaluated by CAP. The extensive study documents significantly reduced dermal thickness and collagen deposition as well as a decrease in both alpha smooth muscle actin-positive myofibroblasts and CD68-positive macrophages in the affected skin in comparison to untreated fibrotic tissue. This study provides the first evidence for the successful use of CAP for treating LS and may be the basis for clinical trials including patients with LS.
https://doi.org/10.3390/biomedicines9111545
Plastische Chirurgie features the Adtec SteriPlas
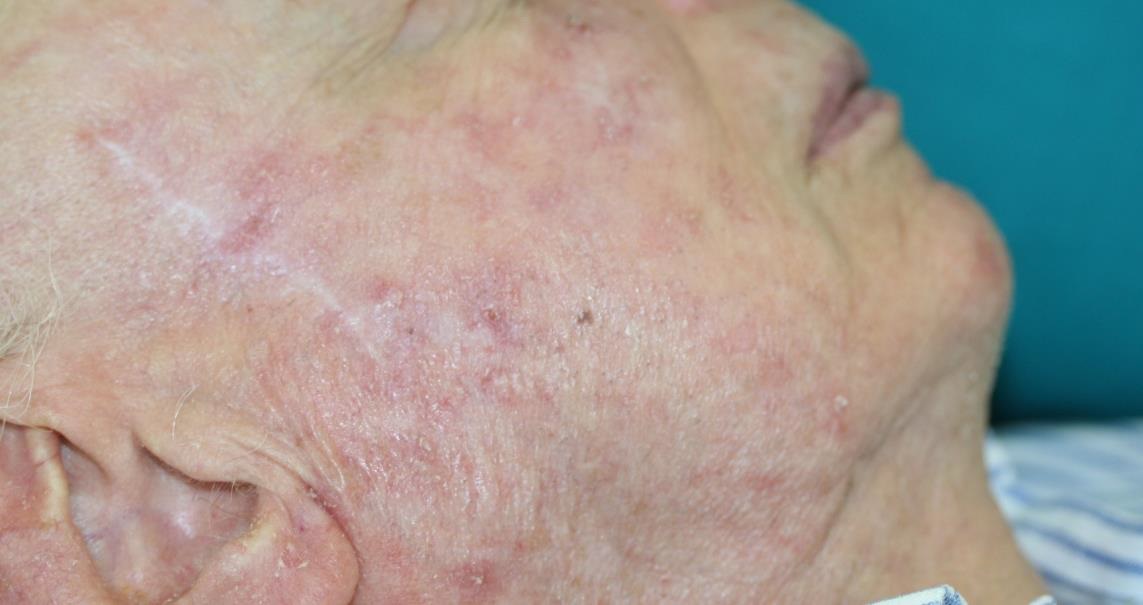
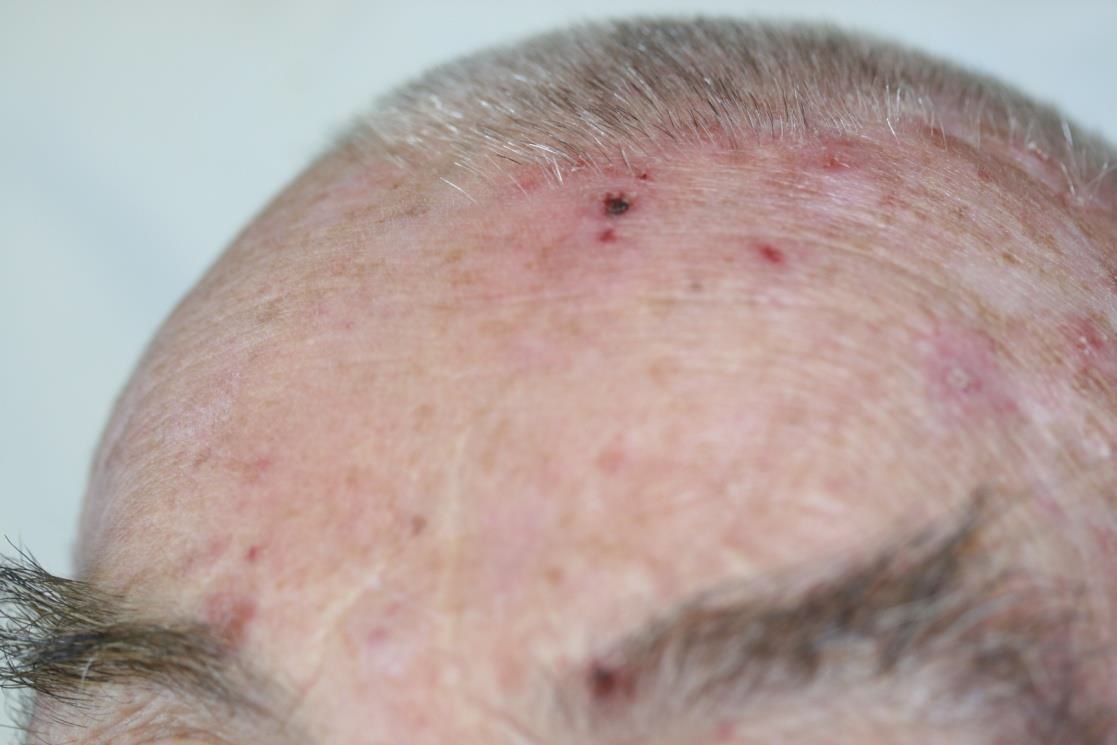
Be sure to read our latest advertorial in the Plastische Chirurgie magazine featuring the Adtec SteriPlas for the treatment of actinic keratoses. Our medical device has been thoroughly tested in clinical trials and studies to prove its efficacy for the treatment of complex and impaired wounds, surgical site infections and medical dermatology conditions leading to accelerated healing.
Existing conventional therapies currently on the market for actinic keratoses have sometimes been shown to bear undesirable side effects. So we are proud to boast the safety and reliability of the Adtec SteriPlas with no side effects reported over continuing use since its existence especially for the treatment of actinic keratoses lesions.
To learn more about our medical device, send us an email at info@adtecplasma.com

The SteriPlas can be used to treat a variety of areas on the body
We are proud have been able to treat a wide scale of body areas. Our clinical evidence ranges across a multitude of conditions and areas of the body including the foot, leg, arms, head, neck, scalp, hands, axilla and even groin. All wounds/lesions present in these areas of the body had shown positive responses to treatment with the Adtec SteriPlas.
We are always open to treating new conditions and welcome any study proposals that you have. If you would like to get the Adtec SteriPlas in your clinic or hospital, please contact us at info@adtecplasma.com where one of our team members will assist you with the delivery.
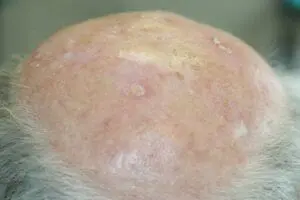
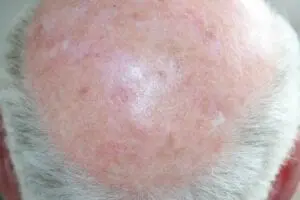
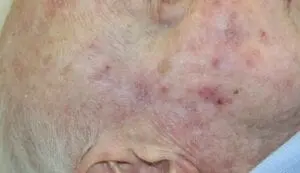
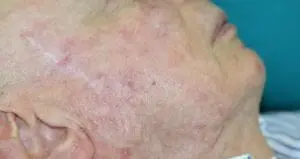
The Adtec SteriPlas featured in another Actinic Keratoses publication
We would like to congratulate Dr Mariachiara Arisi and her team at The Department of Dermatology, Brescia Hospital in Italy for their latest publication: “Cold Atmospheric Plasma (CAP) for the Treatment of Actinic Keratosis and Skin Field Cancerization: Clinical and High-Frequency Ultrasound Evaluation”.
This publication features the Adtec SteriPlas and further demonstrates its efficacy for treating complicated Actinic Keratoses (AK) lesions. All patient enrolled into this study had previously shown resistance or intolerance to conventional field-directed therapies. The results of the study show how all patients who received treatment with the Adtec SteriPlas had significantly reduced AK lesions with the benefit of no side effects observed, quite the opposite for conventional therapies that often bear undesirable side effects. The Adtec SteriPlas continues to be a favoured treatment alternative for actinic keratoses by continuing to be faster-acting, safer and reliable.
You can read the full publication here: https://link.springer.com/article/10.1007%2Fs13555-021-00514-y
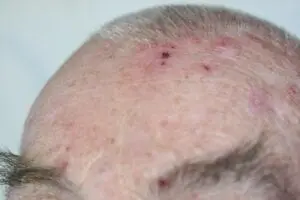
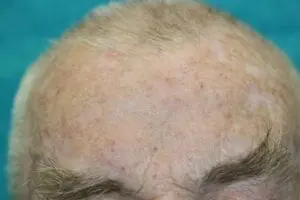
SteriPlas cold plasma successful at killing multi-resistant bacteria
Our animation video shows how bacteria are easily destroyed using our patented cold plasma technology. The components of cold plasma work collectively to target bacteria and physically rupture the structure, allowing micropores to be created which gains access to the microbial DNA to be destroyed. This effectively kills bacteria, even if they are protected within biofilm.
Learn more about our clinical evidence by visiting our webpage www.adtechealthcare.com or send us an email at info@adtecplasma.com
Click the link below to access the animation video:
Animation video to show how the SteriPlas can successfully kill multi-resistant bacteria
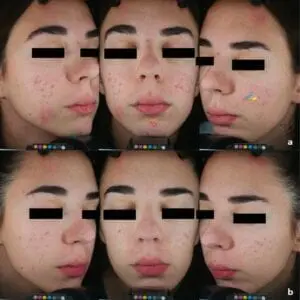
SteriPlas treatment for Acne Vulgaris
We are excited to announce the recent publication, “Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris” which features our Adtec SteriPlas cold plasma for the treatment of acne.
The paper created by Dr Mariachiara Arisi from the Department of Dermatology at the University of Brescia, Italy demonstrates the antibacterial efficacy of our patented cold plasma for the treatment of acne vulgaris patients. It indicates the significant reduction of acne skin lesions in treated patients who were previously unsuccessful treated with topical drugs. Unlike topical drugs, the Adtec SteriPlas demonstrated a safe, effective, and well-tolerated treatment option with no side effects for the treatment of acne patients.
No adverse effects or skin reactions were reported either during the treatment nor at 3-months follow-up. Treatment was completely painless and well tolerated. Patients did not report itching or burning sensation during plasma application and in the following days.
The full paper can be found here: https://www.sciencedirect.com/science/article/pii/S2212816620300172