SteriPlas to be presented at the ISDF conference
We’re looking forward to supporting Dr Pierides’ presentation at The International Symposium on the Diabetic Foot (ISDF) conference this Friday as he presents the strong benefits of our SteriPlas Cold Plasma medical device for the management of chronic Diabetic Foot Ulcers. His presentation, “DGH experience of using cold argon plasma in treating diabetic foot ulcers and its role in antibiotic stewardship” discusses the outcomes of 37 patients all of whom received once weekly SteriPlas treatment. The SteriPlas is shown to eliminate the use of antibiotics for chronic DFUs inhibited by multi-resistant bacteria. The unique physical mode of action that the SteriPlas delivers to the infection site ensures a total destruction of all antimicrobial resistant bacteria even if they are protected within biofilm.
For more information about our clinical evidence and medical device, please contact us at info@adtecplasma.com
Day 2 of EWMA
It's day 2 at EWMA 2023 and we're having a great time meeting with new and existing customers. If you want to learn about the most effective Cold Plasma medical device for woundccare with the most clinical trials, strongest efficacy and no side effects, please visit our booth at no. 116 to see a live demonstration of the SteriPlas in action.


Adtec Healthcare Ltd to showcase at the LSX Conference in London
We’re on a mission to revolutionize the global wound care market! Adtec Healthcare is participating in the LSX Conference in London.
Our managing director, Mary McGovern shall present our cold plasma technology at the MedTech Showcase on Thursday 4th May at 11:30 am.
We have developed SteriPlas, a medical device for the treatment of chronic wounds. The most effective cold plasma technology for wound care treatment, proven by clinical studies. The device uses proven antibacterial cold atmospheric argon plasma to treat chronic wounds, dermatology, and post-operative wound infections. Enables faster healing, reduces hospital stays, eliminates biofilms and the use of antibiotics. CE Marked and available for sale in UK and EU. Efficacy proven in 70+ peer-reviewed clinical studies. Early adoption by leading wound care specialists. Strong IP portfolio, comprising of granted and recently submitted patents covering various aspects of their device.
We are currently speaking to investors to accelerate sales in Europe and for the launch in the US. At LSX, we are meeting investors and partners interested in medical devices and wound care.
Please contact us at info@adtecplasma.com to arrange a meeting.
https://www.lsxleaders.com/lsx-world-congress/agenda/medtech

Thomas presents at the ISHLT show


A fantastic presentation delivered by Thomas Schlöglhofer yesterday at the ISHLT - International Society for Heart and Lung Transplantation show. We hope you got the chance to see his presentation and the promising results he has achieved managing LVAD infections with our Cold Plasma SteriPlas medical device. If you are interested to learn more about the SteriPlas and the benefits it can bring to your patients, please contact us at info@adtecplasma.com
A great start to the ISHLT show


What a fantastic start to the International Society For Heart and Lung Transplantation! We’ve been swamped with your presence and interest. It’s been a pleasure to meet new and old faces and we’ve been excited to share how well our leading Cold Plasma SteriPlas medical device is changing lives in the UK and Europe for LVAD patients.
We look forward to welcoming you to our booth no.416 ready to introduce you to the strong benefits of our Cold Plasma efficacy.
Cold Plasma Walk at ISHLT
We’re excited to be exhibiting at the ISHLT conference next week and we look forward to supporting Mr Thomas Schlöglhofer as he presents his SteriPlas Cold Plasma presentation for the management of LVAD infections during the “Bugs, Drugs, and Thugs: Fighting Infection in VADs Any Way We Can” session on Wednesday 19th April between 5:30 PM - 6:30 PM.
Due to popular demand, we will be hosting a Cold Plasma Walk session at our exhibition booth on Wednesday 19th April during the lunch break. We encourage you to visit our booth no. 416 so you can see Thomas present our medical device and explain the strong benefits it can bring to the management of LVAD infections.
Our proven and patented Cold Plasma medical device has been widely adopted as the solution for LVAD infections due to its unique physical mode of action, quick treatment times and accelerated healing with stalled infections due to biofilm all whilst protecting the patient and user from no side effects.
Contact us at info@adtecplasma.com for more information.
Day 2 of the SCTS

It's Day 2 of the Society for Cardiothoracic Surgery in GB & Ireland (SCTS) conference and we've been overwhelmed with all your interest in our Cold Plasma medical device, the SteriPlas. Visit us at booth 18 to learn more about how to bring the benefits of our medical device to your hospital.
How does the Adtec SteriPlas work so effectively?
Our patented cold plasma therapy utilises a physical mode of action approach delivered from an indirect microwave plasma source. Our cold argon plasma is an electrically charged and ionised gas that consists of charged particles, UV light and reactive oxygen and nitrogen species with the latter created as a by-product. They all work collectively to physically rupture the structure of bacteria even if they are protected within biofilm. Regardless of the type of bacteria, its resistance profile or whether they are Gram-positive or Gram-negative our cold plasma works quickly and effectively to destroy them. This has the leading advantage over conventional therapies such as antibiotics that rely on a chemical mode of action approach.
Although antibiotics have been a pivotal part of today’s medicine, they also contribute towards antimicrobial resistance rates particularly because bacteria can develop resistance such as those observed with diabetic foot ulcers that can often lead to amputations as a result.
However, as well as being fast-acting and effective, our cold plasma has shown that a primary or secondary resistance is unlikely to be developed due to its physical mode of action.
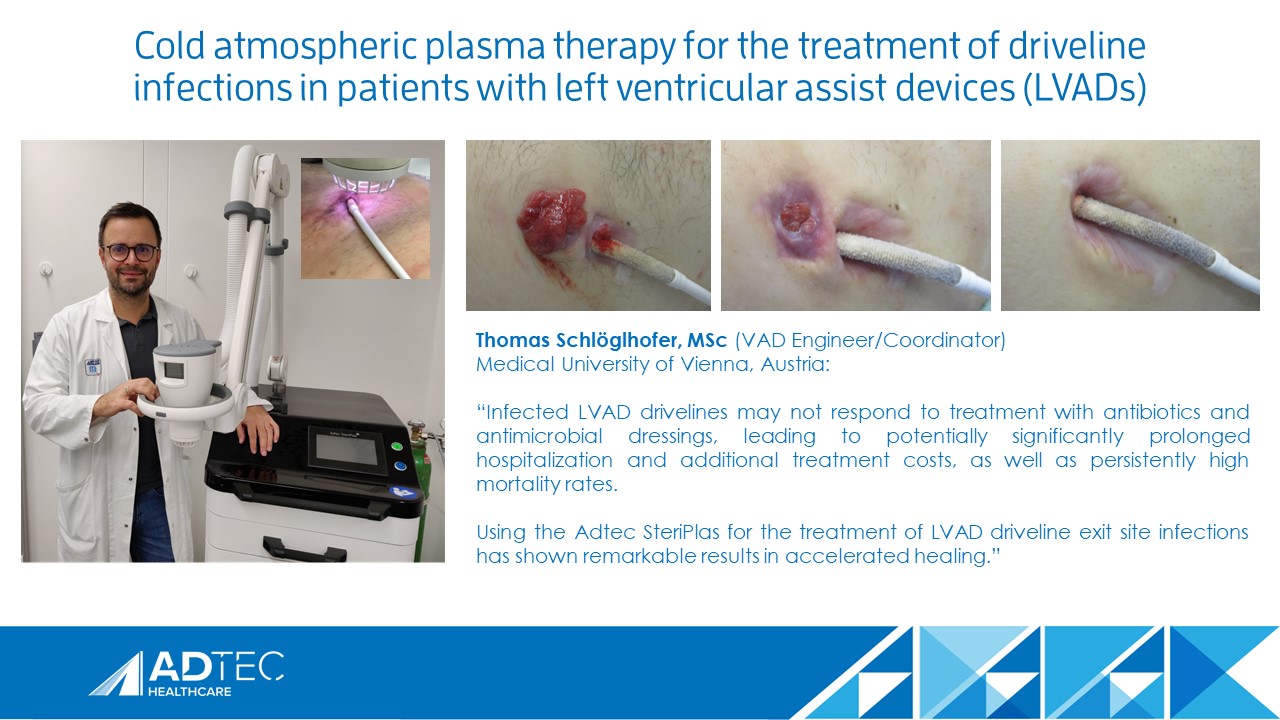
Mr Schlöglhofer to present SteriPlas at the ISHLT
Congratulations to Thomas Schlöglhofer from the Medical University of Vienna for his continued success of using our medical device to treat infections in LVAD patients. He will be presenting at The International Society for Heart and Lung Transplantation (ISHLT) conference in April to deliver the great results he has so far achieved.
His presentation, “Cold Atmospheric Plasma Therapy: A Powerful Tool for Treating Driveline Infections in Left Ventricular Assist Device Patients” will be during the “Bugs, Drugs, and Thugs: Fighting Infection in VADs Any Way We Can” Session on Wednesday 19th April between 5:30 PM - 6:30 PM.
We hope you will be there too to see him deliver his promising results.
For more information on how our medical device is fighting against multi resistant bacteria and biofilm in LVAD patients.

Today at The DGTHT Hermedizin 2023
It was a pleasure to see Dr Rotering’s presentation today at the DGTHT Hermedizin 2023 conference. His presentation on the SteriPlas for the treatment of deep sternal wound infections shows just how effective our Cold Plasma medical device is for the treatment of these chronic and deep wounds infected with biofilm.
Not only has the SteriPlas has shown to significantly decrease hospital bed occupancy, but it has shown to reduce mortality rates and prevent the need for expensive treatments by allowing sternal wall stability. The SteriPlas can successfully kill all forms of bacteria, regardless of their resistance profile or if they are protected within biofilm.