Handheld and Effective

At Adtec Healthcare Limited, innovation never stands still. Building on the success of our flagship SteriPlas Cold Plasma medical device, we're excited to share a glimpse of the PlasmaTact – our next-generation, compact, handheld argon microwave plasma system.
While not yet on the medical market, the PlasmaTact represents our commitment to pushing the boundaries of plasma technology. Designed for versatility, portability, and new applications, this device showcases the future of plasma-based solutions.
Stay tuned as we continue to explore groundbreaking opportunities with the PlasmaTact – because innovation is at the heart of everything we do.
Day 2 of Medica

Day 2 at MEDICA 2024 – Another Fantastic Day for Adtec Healthcare Limited!
Today marked another successful day at the MEDICA - Leading International Trade Fair. We've had the pleasure of connecting with both new and familiar faces, sharing insights, and discussing how our innovative solutions continue to advance healthcare.
Thank you to everyone who stopped by our booth to learn more about what we do. We're excited to keep building strong relationships and exploring opportunities to make a positive impact in the healthcare industry.
Looking forward to more great conversations in the days ahead! See you tomorrow at Hall 15, Booth A24-3.
Day 1 of Medica



Exciting Day at MEDICA 2024 in Düsseldorf!
What an incredible day connecting with industry leaders, innovators, and healthcare professionals here at the Medica exhibition! We’re thrilled with the positive response to our solutions and the inspiring conversations we’ve had with so many of you.
If you’re attending MEDICA, make sure to stop by our booth, A24-3 in Hall 15! Our team is here, ready to answer your questions and showcase how our SteriPlas Premium Cold Plasma can make a difference in healthcare. Don’t miss out on the chance to see what’s new, chat with us, and be part of this inspiring event!
Looking forward to welcoming even more of you tomorrow!
Adtec joins the MedTech World 2024 this week
We’re thrilled to announce our participation in MedTech World 2024 this week!
Join us as our CEO takes the stage for the panel discussion, "How We Do It: Investors and Entrepreneurs Together." This is a fantastic opportunity to explore innovative collaborations that drive the future of healthcare technology.
Stay tuned for insights and highlights from the event! Let’s connect and shape the future of MedTech together!
https://med-tech.world/news/improving-wound-management-adtec-healthcares-steriplas-technology-explained/

First ever recorded study for CAP on Pediatric Cannula infections
🚨 Exciting News! 🚨
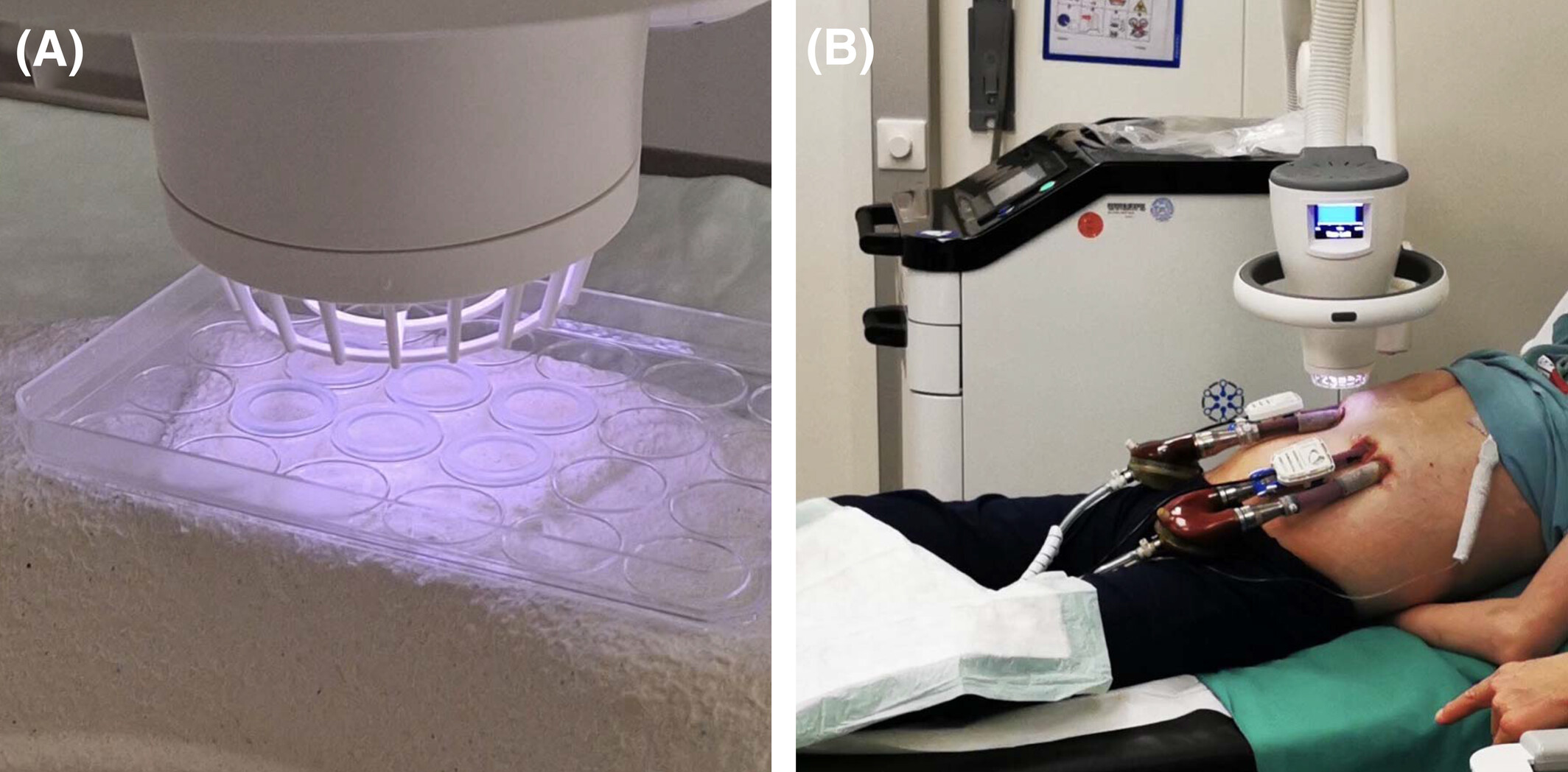
We are thrilled to share a groundbreaking study by Dr. Schlöglhofer, recently published in the Artificial Organs journal from Wiley. This study highlights the remarkable safety and efficacy of using SteriPlas Premium Cold Plasma for managing infections on Berlin Heart EXCOR cannulas.
🌟 This is the first publication to demonstrate the potential of CAP therapy for treating infections and inflammation in pediatric patients with paracorporeal pulsatile VADs.
Key findings show that SteriPlas treatments:
- Do not alter the surface structure of cannulas, as confirmed by SEM micrographs.
- Lead to significant improvements in DESTINE wound staging.
- Drastically reduce bacterial load and inflammatory markers.
- Achieve these results with no observed side effects.
This study marks an exciting step forward in infection management for pediatric VAD patients. To read the full publication, visit: Wiley Library.
Exhibiting at the EUMS 2024 show


Dr Schlöglhofer presents the SteriPlas at ESAO 2024


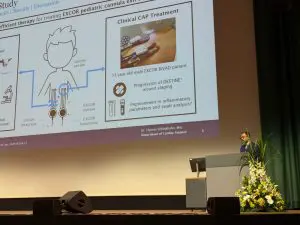
A huge congratulations to Dr Thomas Schlöglhofer for his presentation at the European Society for Artificial Organs 2024 conference.
His presentation, “Cold Atmospheric Plasma Therapy as a Novel Treatment for Berlin Heart EXCOR Pediatric Cannula Infections” features the use of our SteriPlas Premium Cold Plasma for infection management on Berlin Heart EXCOR cannulas in Pediatric patients.
We thank Dr Schlöglhofer for his work towards this study and for recommending the SteriPlas as the only Cold Plasma device for the infection management of Berlin Heart EXCOR products.
Presentation at the DFSG 2024


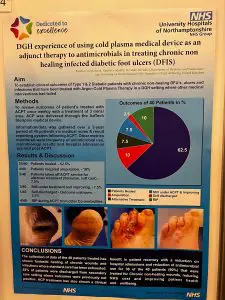
We enjoyed watching Jemma present on behalf of Kettering General Hospital NHS for the SteriPlas treatment of chronic diabetic foot ulcers at the Diabetic Foot Study Group (DFSG). Our Premium Cold Plasma has proven efficacy and anti-biofilm properties essential for the healing of these complex wounds.
Day 2 of the DFSG 2024


Glad to be at the Diabetic Foot Study Group (DFSG) conference catching up with existing customers and to those new who have learnt the benefits of our SteriPlas Premium Cold Plasma. We look forward to the Kettering NHS presentation later today featuring the SteriPlas efficacy for chronic Diabetic Foot Ulcers.
Exhibiting at the DFSG 2024