Diabetic Foot Ulcer Health Economics

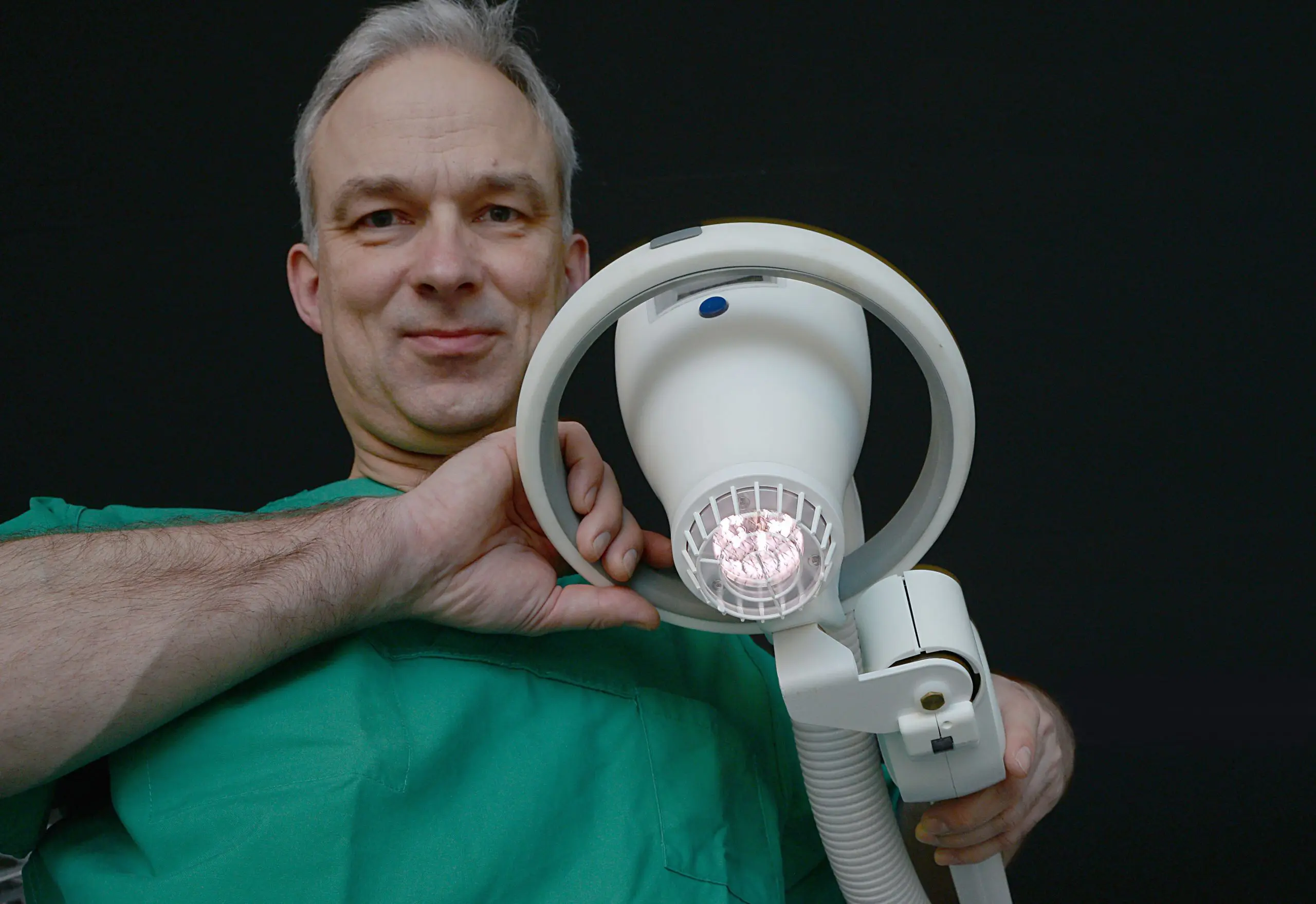
Adtec Healthcare Limited has invested over 10 years in clinical trials and studies to bring you over 80+ peer reviewed studies to validate why the SteriPlas Premium Cold Plasma is the leading medical device in the medical industry for chronic infection management. Not only is our medical device clearly proven to be the strongest against multi-resistant bacteria protected within biofilm but it can treat a wide variety of deep and hard to reach areas that are often recalcitrant to conventional therapies, therefore not limited to superficial wound treatments.
Our recent goal has been to collect the cost effectiveness findings to demonstrate just how the cost burden can be eliminated by the hospital when using our SteriPlas. In the case of chronic diabetic foot ulcers with biofilm presence in the NHS, the SteriPlas has been shown to reduce antibiotic usage by 57% for patients thus relieving the ongoing costs thrown onto the hospital service. Not only are patients healed significantly faster, bed occupancy significantly reduced, and limbs are saved but there is a significant reduction in patient visits to the hospitals thus relieving further costs associated to the hospital.
For more information about our Health Economics data, contact us at info@adtecplasma.com
SteriPlas to be presented at the DFSG conference
Adtec looks forward to supporting Ms Jemma Cruickshank, Antimicrobial Pharmacy Technician from Kettering General Hospital NHS Foundation Trust, at the Diabetic Foot Study Group (DFSG) conference in September.
Jemma has been invited to present the “Retrospective review of the use of argon cold plasma therapy in non-healing diabetic foot ulcers over a 3-year period within a DGH Diabetic Foot MDT Service” at the conference.
Her presentation will include the strong benefits of the SteriPlas on chronic and large diabetic foot ulcers prone to biofilm infections.
The abstract for this presentation can be found here: https://distribute.m-anage.com/from.storage?image=erIsXlaDuNOv64Tv4JyKY6XW3cI1HigkZNgT5cOgYv7Vzt_UHvGbDPesijzVroVO0
For more information about our SteriPlas Premium Cold Plasma medical device, send us an email info@adtecplasma.com
What makes our SteriPlas safe and effective?

What makes our SteriPlas Premium Cold Plasma safe and effective?
Adtec has been the global leader of Cold Plasma medicine, having developed Cold Plasma products for over 20 years and being the first company worldwide to conduct clinical trials on wounds. In total, Adtec boasts over 80+ clinical trials and studies with no side effects reported.
Whilst there are many forms of Cold Plasmas on the market, our SteriPlas Cold Plasma is dense and slowly propelled towards the treatment site enabling bacterial destruction in deep and hard to reach infection areas. The argon Cold Plasma is microwave, 2.45GHz generated using crucial components within the body of the medical device. This is why it is larger in size but at the advantage of delivering reliable and consistent results and easily tackles complicated biofilm issues within chronic infections unlike the other variations of Cold Plasma on the market. This is also why our clinical evidence is often cited by other Cold Plasma manufacturers as our promising results far outweigh the results achieved by Cold Plasma devices with different plasma designs.
To learn more about our strengths and benefits, contact us at info@adtecplasma.com
Leading the way in Cold Plasma Medicine
The antibiofilm properties of the SteriPlas has been widely documented across our 80+ clinical trials and studies. It is being widely used to treat a multitude of infection conditions such as Diabetic Foot Ulcers, Sternal Wounds and LVAD infections in the UK, EU and SE Asia. Helping to significantly reduce morbidity, mortality, amputation and bed occupancy rates, the SteriPlas has been transforming modern medicine by simplifying and accelerating the way these chronic and non-healing wounds are treated. We have also documented the significant cost saving benefits of using the SteriPlas Premium Cold Plasma all with the added benefit of no side effects due to its safe, microwave 2.45GHz argon plasma generation.
To learn more about the benefits we can bring you, contact us at info@adtecplasma.com
Upcoming Exhibitions for 2024


Adtec looks forward to exhibiting at the Diabetic Foot Study Group (DFSG) conference between 6-8 September and the European Mechanical Support Summit (EUMS) conference between 11-14 September. At both conferences, we will have our SteriPlas Premium Cold Plasma medical device on our booth for a live demonstration. The SteriPlas boasts a strong clinical evidence collection of 80+ clinical trials and studies across a multitude of infection conditions.
To better understand our medical device and the positive imprint we have made on the severe infection conditions, please visit our booth during the two shows to see the SteriPlas in person.