Our Presentation video from the LSI Emerging Medtech Conference
Last month our CEO had presented the strong antibacterial benefits, clinical evidence and our business plan for our Premium Cold Plasma at the Life Science Intelligence (LSI) Emerging Medtech Conference.
After much positive interactions since the LSI which has led to promising meetings, we share with you that presentation : https://fb.watch/nWbLL1E_8d/
Adtec Microwave Argon Plasma has proven clinical efficacy in chronic infection management compared to standard care.
Adtec’s patented microwave argon plasma was designed specifically to create a denser safe plasma to manage infection with proven clinical efficacy over standard treatments. Our Plasma blend is effective at treating bacteria enclosed within biofilm and reaching hard to treat areas e.g. along drivelines. This clinical evidence has been presented at conferences and published in over 80 peer-reviewed publications.
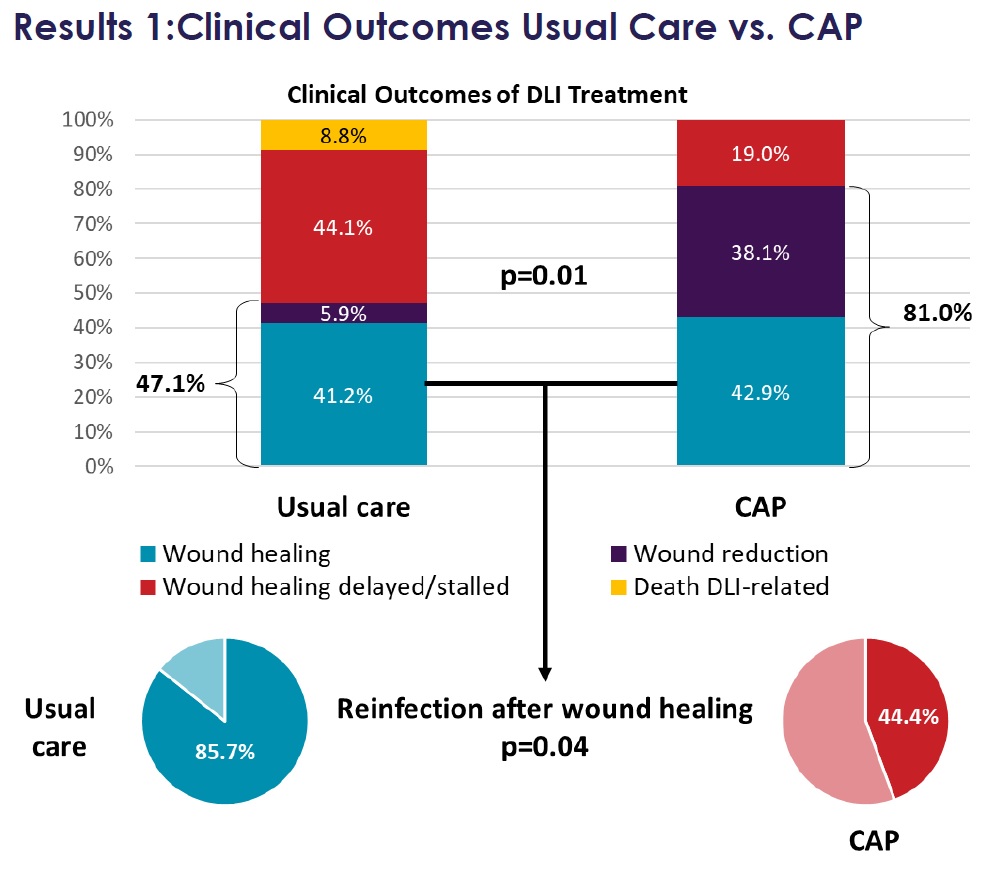
The recent clinical evidence featured in Mr Thomas Schlöglhofer’s data illustrates the advantages of our SteriPlas medical device for deep LVAD infections. This showed Adtec Cold Plasma had 81% efficacy at wound management vs 47.1% efficacy with standard of care.
“Cold Atmospheric Plasma Therapy as an Effective Treatment of Left Ventricular Assist Device Driveline Infections” at the EACTS European Association for Cardio-Thoracic Surgery conference in Vienna (October 2023) presented by Mr Thomas Schlöglhofer, the Department of Cardiac Surgery, Medical University of Vienna.
Clinical Trials conducted using Adtec microwave argon plasma results include a 73.5% reduction in bacterial load with standard of care plus plasma treatment compared to a 50% reduction with standard of care alone. This represents a 47% increase in bacterial load reduction with SOC and plasma compared to SOC. All 24 patients received standard wound care to all wounds; 22 patients also received systemic antibiotics (92%).
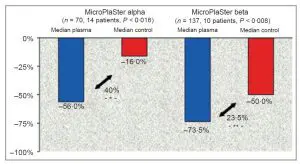
Successful and Safe Use of 2 Min Cold Atmospheric Argon Plasma in Chronic Wounds: Results of A Randomized Controlled Trial. Isbary, G., et al British Journal of Dermatology, 2012. 167(2)