The Safety and Reliability of our Cold Plasma

Is our cold plasma safe?
Yes!
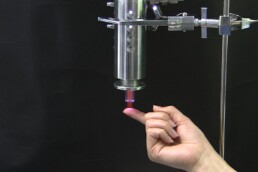
As you can see from the photo, our Adtec technician demonstrates touching the cold plasma jet.
An extensive list of practical tests and experiments were conducted during our first stage, pre clinical studies and ongoing post-market studies. This allowed us to carefully develop a cold plasma medical device that could bring all the antibacterial benefits but at the same time remain painless and contact-free so that it could be well tolerated in patient treatments. We were after all the first company worldwide to introduce cold plasma treatment on wounds demonstrated in our clinical trials.
As cold plasma can be generated through a variety of ways, they are also dependent on the plasma source design and surrounding environment. The components of plasma include reactive oxygen and nitrogen species and UV that need to be controlled to ensure they are within safe levels for medical use. Some plasma devices may also deliver fluctuating inconsistent cold plasma output as they are sensitive to changes in humidity and temperature in the treatment room. This may often result in undesirable side effects.
Because the Adtec SteriPlas is a low energy, microwave-powered, cold atmospheric argon plasma medical device this means its safety remains the main priority in line with delivering repeatable and successful results. It is also the reason why we are proud to state our medical device has NO side effects associated to it.
Our patented 6 electrode Cold Plasma Torch

The Adtec SteriPlas features a patented 6 electrode cold plasma torch. It’s proven efficacy for the destruction of all forms of bacteria including multi-resistant bugs makes it an alternative to antibiotic therapy for the treatment of wounds, surgical site infections and medical dermatology skin conditions. This is due to the unique physical mode of action delivered with our cold plasma as opposed to the chemical mode of action demonstrated with antibiotics. Broader studies have shown that antimicrobial resistance is unlikely to be generated with cold plasma treatment because of this.
A huge collection of our peer reviewed publications documents the clinical efficacy and safety of our medical device across a broad range of conditions including diabetic foot ulcers, left ventricular assist device (LVAD) infections, deep sternal wound infections, actinic keratoses and acne.
To learn more about our medical device or to obtain one in your clinic practice, please contact us at info@adtecplasma.com
Characterisation of a Cold Atmospheric Pressure Plasma Torch for Medical Applications

We congratulate Dr Adam Bennett on his most recent publication, “Characterisation of a Cold Atmospheric Pressure Plasma Torch for Medical Applications: Demonstration of Device Safety”.
The safety and effectiveness of plasma devices are of crucial importance, especially for applications where the plasma is discharged near humans. This study presents the novel design and characterisation of a Cold Atmospheric Plasma torch (SteriPlas), which is used in medical applications. The study shows the characterisation methodology that must be undertaken to show that a plasma device is safe, especially when used in an application on human skin. The emission spectrum discharged from the plasma torch is shown. The UV emitted is measured and the effective irradiance is calculated. The effective irradiance enables the determination of the maximum UV exposure limits, which in this application are shown to be over two hours; however, in some applications may be only seconds. NOx and ozone emissions are also recorded. The NOx levels in this application are shown to be orders of magnitude lower than their safety limits and the ozone emissions are also shown to be safe; however, in some plasma technologies the NOx and ozone levels are orders of magnitude higher than the safe levels.
This paper concludes with a discussion of how safety limits vary in different regions around the world and proposes an international standard. It documents the safety of our medical device which further reiterates one of our main strengths where no side effects have been reported.
Access to the full paper can be found here: https://www.mdpi.com/2076-3417/11/24/11864
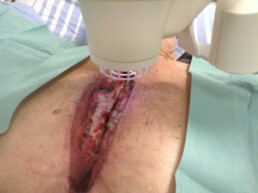
Deep Sternal Wound Infections (DWSI) and the Adtec SteriPlas

Unlike other cold plasma medical devices currently present on the market, the Adtec SteriPlas is not limited to the treatment of superficial wounds only.
For example, deep sternal wound infections (DSWIs) are a potentially devastating complication of median sternotomy performed in cardiac surgery. These large wound cavities are prone to infections and there is a concerningly high morbidity and mortality rate associated to these patients. The use of conventional therapies alone has shown to not always be successful and expensive muscle flap surgery is usually required.
The large 12cm2 treatment area of the Adtec SteriPlas covers a greater surface area of the wound compared to its competitors. This allows the benefit of a quick 2 minute treatment time for every 12cm2 covered. Because of its propulsion towards the treatment site, the cold plasma components work quickly to defeat bacteria and allow the management of the infection. The use of the Adtec SteriPlas has shown to accelerate healing rates and prevent the need for expensive surgery options that would otherwise have been needed.
It has been praised as a lifesaving medical device and is now widely used across multiple hospitals for the management of DWSIs.
Promising, consistent and reliable results achieved with the Adtec SteriPlas



Whilst Left ventricular assist devices (LVADs) have become essential mechanical pumps for patients who have had end stage heart failures, they are also prone to infections especially with the presence of biofilm that complicates treatment options. Cardiologists often rely on conventional antibiotic therapy but with rising antimicrobial resistance rates, they sometimes offer little to no help in achieving stabilisation.
The strong properties of the Adtec SteriPlas cold plasma has been proven to offer complete stabilisation of the LVAD system by destroying all forms of multi-resistant bacteria present, even if they are protected within biofilm. This is due to the unique cold plasma properties that can travel through the gaps around and down the driveline to reach the site of biofilm. This area would normally have been difficult to treat with conventional therapies such as antimicrobial dressings as they are hard to reach, but the Adtec SteriPlas has shown to travel to these sites with ease due to its remarkable antibacterial gaseous properties.
To learn more about our strong collection of clinical evidence, please contact us at info@adtecplasma.com to learn more
LVAD - Heidelberg publication 2021
Congratulations to Dr Kremer and the Cardiology Team at The University Hospital Heidelberg for their new publication, “Follow-Up after Cold Atmospheric Argon Plasma for Wound Management of Driveline Infections - A Promising Strategy”. This publication features the use of our Adtec SteriPlas medical device for the treatment of driveline infections (DLI). The results demonstrate how the safety, reliability and painless treatment from the Adtec SteriPlas can significantly reduce the wound and infection around the DLI, with the opportunity for sustainable freedom from DLI.
Surgical site infections treated with the Adtec SteriPlas
Surgical site infections (SSIs) are still a severe complication after cardiac surgery with a mortality estimated range of up to 40% of cases. These post-operative wounds are generally associated with longer hospital stay and the delayed or inadequate therapeutic measures can lead to avoidable hospital costs for the healthcare system. The significant cost burden on the hospital for a single SSI case is €36,000. 1
It has already been shown that the Adtec SteriPlas is effective due to its increased penetration depth in SSIs to defeat bacteria even under a layer of biofilm independently to their resistance profile. This includes multi-resistant bacteria that have shown little or no response to antibiotics previously. In combination with advanced negative pressure wound treatment (aNPWT), the Adtec SteriPlas has been praised as a “tissue saving approach” offering patients a favourable treatment method for these complicated and chronic wounds compared to conventional treatment therapies. 2
Studies conducted using the Adtec SteriPlas have shown a significant decrease in the mortality rate coupled with accelerated healing. Patients with infected drivelines such as those with left ventricular assist devices have healed in as quick as 1 week, and patients with sternal SSIs have healed in as quick as 6 days. 2,3

Both patients and doctors have praised the Adtec SteriPlas for its exceptional ability at delivering promising results leading to healing.
Be sure to contact us for more information and to see how our medical device may benefit your patients.

- H. Rotering, Cold atmospheric plasma – New options for infection control in wound management, EWMA 2016.
- H. Rotering, Cold atmospheric plasma and advanced Negative Pressure Wound Treatment – First results of a tissue saving approach for deep surgical site infections, EACTS 2018.
- H. Rotering, Das infizierte Implantat, Komplexe Wundbehandlung – Kaltes atmospherisches Plasma und advanced NPWT in der Herzchirurgie, Chirurgie 2019.