Antimicrobial (antibiotic) Resistance – the drugs won’t work !
Antimicrobial resistance (AMR) poses a significant global threat of far-reaching proportions. It is estimated that drug resistant infections contribute to nearly 5 million deaths every year and predicted to increase to over 10 million deaths every year. WHO has declared that AMR is one of the top 10 global public health threats facing humanity.
(The worldwide total deaths from COVID is now just over 6 million and we all know how frightening it was before the vaccine was developed)
AMR occurs when bacteria, viruses, fungi and parasites change over time and no longer respond to medicines making infections harder to treat and increasing the risk of disease spread, severe illness and death. As a result, the medicines become ineffective and infections persist in the body, increasing the risk of spread to others. Microorganisms that develop antimicrobial resistance are sometimes referred to as “superbugs”. Without effective antimicrobials, the success of modern medicine in treating infections, including during major surgery and cancer chemotherapy, would be at increased risk. Chronic infections if left untreated could result in tissue damage, amputation, longer stays in hospitals, surgical interventions, or increased possibility of mortality. Patients who are infected with drug-resistant infections are more likely to develop complications and are up to three times more likely to die from the infection. Non-healing wounds in particular, are characterised by complex and mixed bacterial populations, often involving antibiotic-resistant bacteria as well as phenotypically tolerant bacteria in biofilm form. The biofilm factor is clearly of considerable clinical importance: it protects bacteria from antimicrobial agents leading to persistent and difficult to treat chronic infections, and it exacerbates the spread of antibiotic resistance. Surgical Site Infections are also linked to anti-microbial resistance.
SteriPlas cold plasma technology kills bacteria by a physical mode of action and bacteria are therefore unlikely to develop primary or secondary resistance, which we have documented from our clinical studies. SteriPlas cold plasma also kills antibiotic resistant bacteria (e.g. MRSA) and kills bacteria encased in biofilm which are typically up to 1000 times more resistant to antibiotics. SteriPlas has proven clinical efficacy in treating wound infections, diabetic foot infections and surgical site infections in all clinical studies, all with the bonus of no side effects reported.
SteriPlas cold plasma can be used to treat topical infections reserving antibiotics for severe systemic infections.
References
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02724-0/fulltext
https://www.worldometers.info/coronavirus/
https://www.who.int/health-topics/antimicrobial-resistance
https://healthfirsteurope.eu/wp-content/uploads/2020/11/A3A4-48pp-Booklet-Spreads-1.pdf
Bowler, P., Murphy, C. & Wolcott, R. Biofilm exacerbates antibiotic resistance: Is this a current oversight in antimicrobial stewardship?. Antimicrob Resist Infect Control 9, 162 (2020).
Diabetic Foot Study Group Conference
Are you excited as we are?
Looking forward to resuming exhibition season at the Diabetic Foot Study Group conference in Slovakia.
We'll be exhibiting between 16-18 Sept with our Adtec SteriPlas on demonstration ready to show you just how quick and effective it is to use and combat antibiotic resistant bacteria within diabetic foot ulcers.

Welcoming delegates at The Malvern Diabetic Foot conference




We’re having a great time welcoming you all to our booth at the Malvern Diabetic Foot Conference, introducing you all to the benefits of the SteriPlas cold plasma for the treatment of diabetic foot ulcers.
For those wishing to learn more about our clinical efficacy in diabetic foot ulcers, please contact us at info@adtecplasma.com for more information.
Welcoming Cardiothoracic Specialists at The SCTS



We're having a great time at the Society for Cardiothoracic Surgery in GB & Ireland conference, introducing the strong benefits of our Adtec SteriPlas cold plasma for deep sternal wound infections.
Please do pop by & see us at booth 18 to see our medical device in action.
The ISHLT Award Goes to Thomas Schlöglhofer!
Congratulations to Thomas Schlöglhofer and his team at the Medical University Hospital in Vienna for winning the ISHLT (International Society for Heart and Lung Transplantation) grant award, sponsored by Abbott and Medtronic.
This prestigious award given to Thomas is for his fantastic work using our Adtec SteriPlas medical device for the successful healing of LVAD Infections.
We look forward to continuing to support Thomas and his team and their presentation of the study at the ISHLT 2023 conference next year.


Next week welcomes the start of the exhibition season
Find us next week at the Society for Cardiothoracic Surgeons in Belfast from 8-10 May. We'll be at booth 18 ready to welcome you and introduce the benefits of #coldplasma in cardiothoracic #infection conditions.
From 10-13 May, we will also be exhibiting at the Malvern Diabetic Foot Conference looking forward to seeing regular faces from this prestigious conference.
We will have our Adtec SteriPlas medical device on our booths, ready to give you a live demonstration of how effective cold plasma is at killing bacteria protected within biofilm.

![]()
Taiwan Wound Therapy Society Conference, March 2022




Sharing some photos from last weekend at the Taiwan Wound Therapy Society conference.
Our medical distributor, SG Biomedical, had a productive weekend demonstrating our SteriPlas to doctors and nurses keen to learn about the benefits of cold plasma for the treatment of wounds, surgical site infections and medical dermatology.
For more information about the Adtec SteriPlas and how to obtain this in the SE Asia region, please contact our distributor SG Biomedical for more information.
ToGCPiCPT

Congratulations to our Business Development Manager, Jeiram, for his published chapter in the “Textbook of Good Clinical Practice in Cold Plasma Therapy”. This prestigious book houses powerful material from esteemed authors in the cold plasma medicine field.
Jeiram’s chapter, “SteriPlas® and PlasmaTact®”, discusses the importance of Adtec Healthcare’s influence in the cold plasma medicine field from being the first company ever to conduct cold plasma clinical trials on wounds and to our modern day clinical efficacy in wounds, surgical site infections and medical dermatology skin conditions.
You can view this chapter here: https://doi.org/10.1007/978-3-030-87857-3_18
For more information about our cold plasma medical and non-medical devices, please contact us at info@adtecplasma.com
Our Cold Plasma Medical Device leads the way
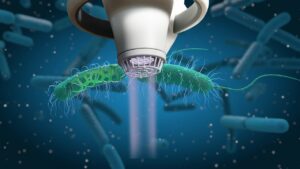
Bacteria protected within biofilm are up to 1000x more resistant to antibiotics. This can complicate treatment options for wounds and surgical site infections but as it is an international form of therapy, it remains used throughout patient treatments despite it offering little to no help in healing problematic/stalled wounds and infections.
The cost burden associated to ongoing antibiotic therapy is significant as is the antimicrobial resistance rates that are ever growing. The World Health Organisation recognizes this and more hospitals are reaching out to alternative therapies to help curb the spending costs and antimicrobial resistance rates.
As the Adtec SteriPlas cold plasma has been shown to kill all forms of bacteria, regardless of their resistance profile or if they are Gram -ve or +ve or even if they are protected within biofilm, it has become widely adopted as an alternative to antibiotic therapy specifically in cases where patients are at a severe stage of infection such as those associated to diabetic foot ulcers and left ventricular assist device infections.
It has been praised as a life-saving medical device helping to heal infections that have been stalled for a very long time where conventional therapies have failed to act, reduce amputation and mortality rates.
This year we look forward to releasing a series of Health Economic publications documenting the cost effectiveness of our medical device against the use conventional therapies. We look forward to releasing this information to you in due time.
Until then, please reach out to us to learn more about why the interest in our medical device is growing daily. The data collection of our safety and efficacy shows why using an Adtec SteriPlas could completely change the way you treat patients.
The Safety and Reliability of our Cold Plasma

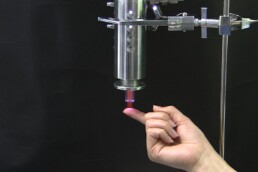
Is our cold plasma safe?
Yes!
As you can see from the photo, our Adtec technician demonstrates touching the cold plasma jet.
An extensive list of practical tests and experiments were conducted during our first stage, pre clinical studies and ongoing post-market studies. This allowed us to carefully develop a cold plasma medical device that could bring all the antibacterial benefits but at the same time remain painless and contact-free so that it could be well tolerated in patient treatments. We were after all the first company worldwide to introduce cold plasma treatment on wounds demonstrated in our clinical trials.
As cold plasma can be generated through a variety of ways, they are also dependent on the plasma source design and surrounding environment. The components of plasma include reactive oxygen and nitrogen species and UV that need to be controlled to ensure they are within safe levels for medical use. Some plasma devices may also deliver fluctuating inconsistent cold plasma output as they are sensitive to changes in humidity and temperature in the treatment room. This may often result in undesirable side effects.
Because the Adtec SteriPlas is a low energy, microwave-powered, cold atmospheric argon plasma medical device this means its safety remains the main priority in line with delivering repeatable and successful results. It is also the reason why we are proud to state our medical device has NO side effects associated to it.