The Adtec SteriPlas is a topical antibacterial, cold plasma medical device with proven antibacterial efficacy, proven accelerated healing and a greater advantage over antibiotics for the treatment of wounds, surgical site infections and dermatological conditions. It has been thoroughly tested in an array of clinical trials and publications to demonstrate its clinical efficacy with no side effects reported.
The Adtec SteriPlas is a broad spectrum antibacterial (including multi-resistant bacteria) with a unique physical mode of action delivered during treatment. Our studies have shown that antimicrobial resistance is unlikely to be developed.
What conditions have been treated so far?
Our Clinical Trials
Efficacy of cold atmospheric plasma vs. diclofenac 3% gel in patients with actinic keratoses: a prospective, randomized and rater-blinded study (ACTICAP)
F. Koch, K.A. Salva, M. Wirtz, E. Hadaschik, R. Varaljai, D. Schadendorf, A. Roesch (JEADV 2020)
https://doi.org/10.1111/jdv.16735
University Hospital Essen, Germany
Status: Trial completed in 2019 and published in 2020.
Total number of patients in study: 60
Pilot Study on the influence of cold atmospheric plasma on bacterial contamination and healing tendency of Chronic wounds
M. Moelleken, F. Jockenhöfer, J. Dissemond
University Hospital Essen, Germany
Status: Trial completed in 2018 and published in 2020
Total number of patients in study: 37
The efficacy of non-thermal gas plasma in the treatment of diabetic foot ulcers stalled by subclinical, biofilm-related wound infection
C. Wiegand, L. Rudtke, K. Cutting, P. Chadwick, S. Haycocks, D. Russell, N. Dewhirst, J. Woods, S. Jeffery
Salford Royal Foundation NHS Trust, UK
Leeds Teaching Hospitals NHS Trust, UK
Status: Trial completed in 2019 and published in 2023
Total number of patients in this study: 15
Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: Results of an open retrospective randomized controlled study in vivo
Isbary G, et al (2013), http://dx.doi.org/10.1016/j.cpme.2013.06.001
Hospital Munich Schawbing, Germany
Status: Trial completed in 2013 and published
Total number of patients in study: 70
Successful and Safe Use of 2 Min Cold Atmospheric Argon Plasma in Chronic Wounds: Results of A Randomized Controlled Trial
Isbary G, et al (2012), https://doi.org/10.1111/j.1365-2133.2012.10923.x
Hospital Munich Schwabing, Germany
Status: Trial completed in 2012 and published
Total number of patients in study: 24
A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients.
Isbary G, et al (2010), https://doi.org/10.1111/j.1365-2133.2010.09744.x
Hospital Munich Schwabing, Germany
Status: Trial completed in 2010 and published
Total number of patients in study: 36
Randomised placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites
S. Karrer, G. Isbary et al (2013)
University Hospital Regensburg, Germany
Status: Trial completed in 2012 and published
Total number of patients in study: 40
Randomized placebo-controlled clinical trial showed cold atmospheric argon plasma relieved acute pain and accelerated healing in herpes zoster
Isbary G, et al. Clinical Plasma Medicine (2014), http://dx.doi.org/10.1016/j.cpme.2014.07.001
Hospital Munich Schwabing, Germany
Status: Trial completed in 2014 and published
Total number of patients in study: 37
Wounds
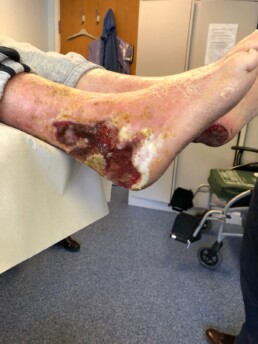
The Adtec SteriPlas has clinical efficacy for the treatment of all wound types. Chronic and complex wounds such as diabetic foot ulcers are often stalled by biofilm. Due to angiopathy and neuropathy conditions, poor arterial flow may mean that antibiotic delivery can play very little to no effect to the healing of these wounds. Sadly, the conditions of these wounds can deteriorate leading to the need of an amputation. The cost burden of managing these wounds can also be expensive if using standard treatments alone:
- £7,800 cost per patient for the treatment of diabetic foot ulcer using standard treatment methods over 12 months.
- £16,900 cost per amputation for diabetic foot ulcer patient over 12 months.
The Adtec SteriPlas has already been postulated as an alternative to antibiotics. Due to its unique physical mode of action delivered during treatment, bacteria can be easily destroyed without developing resistance to our patented cold plasma. This gives it the treatment advantage over conventional therapies. Treating diabetic foot ulcers with the Adtec SteriPlas has shown to accelerate healing as well as be favoured by patients for being contact-free and painless. It has also shown to prevent the need for amputations and save lives. Treatment with the Adtec SteriPlas is well tolerated and no side effects have been reported.
Infected Drivelines
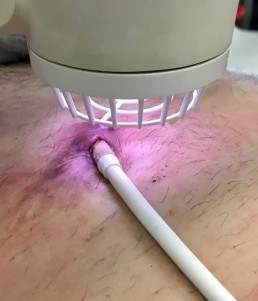

Infected drivelines may show recalcitrance to treatment with antibiotics and antimicrobial dressings. Antimicrobial dressings are unable to wrap the around infected driveline towards the source of biofilm.
Using the Adtec SteriPlas for the treatment of surgical site infections has shown remarkable results in accelerated healing. The gas plasma produced can penetrate through the gaps in crevices around the infected driveline reaching towards the source of biofilm where the bacteria is then destroyed using a physical mode of action.
Deep sternum infections are also very complex cardiac wound infections as a result of heart surgery. The mortality rate of patients with sternum infections are relatively high. The Adtec SteriPlas has been documented as a tissue and life saving approach to treating these complex wounds, eliminating the need for omentum majus or muscle flap plastic surgery. Treatment with the Adtec SteriPlas has shown to accelerate healing in problematic and stalled deep sternum infections or infected drivelines that are infected with biofilm. The treatment is also well tolerated with no side effects being reported.
Deep Sternal Wound Infections
Deep Sternal Wound Infections (DSWIs) are infected wounds derived from complications of cardiac surgery. These are typically treated with antibiotic therapy but remain a severe problem in cardiothoracic surgery with typically a high mortality rate.
Due to exposure of the internal chest cavity that significantly increases the infection rate, it is imperative to heal these patients as quickly as possible by least invasive means necessary. As expected, antibiotics are used but in the presence of multi-resistant bacteria, this may play little to no effect.
The Adtec SteriPlas has shown to successfully treat these patients by eliminating the infection present.
This offers a new treatment approach and is a tissue saving approach and prevents the need for expensive and complex omentum majus or muscle flap plastic surgery.
Average wound closure time = 16 days of treatment.